Label: ORGOVYX- relugolix tablet, film coated
- NDC Code(s): 72974-120-01, 72974-120-95, 72974-120-97
- Packager: Sumitomo Pharma America, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated October 31, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ORGOVYX safely and effectively. See full prescribing information for ORGOVYX.
ORGOVYX (relugolix) tablets, for oral use
Initial U.S. Approval: 2020INDICATIONS AND USAGE
ORGOVYX is a gonadotropin-releasing hormone (GnRH) receptor antagonist indicated for the treatment of adult patients with advanced prostate cancer (1).
DOSAGE AND ADMINISTRATION
- Recommended Dosage: A loading dose of 360 mg on the first day of treatment followed by 120 mg taken orally once daily, at approximately the same time each day (2.1).
- ORGOVYX can be taken with or without food (2.1, 12.3). Instruct patients to swallow tablets whole and not to crush or chew tablets (2.1).
DOSAGE FORMS AND STRENGTHS
- Tablets: 120 mg (3).
CONTRAINDICATIONS
Known severe hypersensitivity to relugolix or to any of the product components (4).
WARNINGS AND PRECAUTIONS
- QT/QTc Interval Prolongation: Androgen deprivation therapy may prolong the QT interval (5.1).
- Hypersensitivity: ORGOVYX can cause hypersensitivity reactions, including angioedema. Withhold ORGOVYX in patients who experience symptoms of hypersensitivity. Discontinue ORGOVYX for severe hypersensitivity reactions and manage as clinically indicated (5.2).
- Embryo-Fetal Toxicity: ORGOVYX can cause fetal harm. Advise males with female partners of reproductive potential to use effective contraception (5.3, 8.1, 8.3).
ADVERSE REACTIONS
The most common adverse reactions (≥ 10%) and laboratory abnormalities (≥ 15%) were hot flush, glucose increased, triglycerides increased, musculoskeletal pain, hemoglobin decreased, alanine aminotransferase (ALT) increased, fatigue, aspartate aminotransferase (AST) increased, constipation, and diarrhea (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Sumitomo Pharma America, at 1-833-696-8268 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
P-gp Inhibitors: Avoid co-administration. If unavoidable, take ORGOVYX first, separate dosing by at least 6 hours, and monitor patients more frequently for adverse reactions (2.2, 7.1).
Combined P-gp and Strong CYP3A Inducers: Avoid co-administration. If unavoidable, increase the ORGOVYX dose to 240 mg once daily (2.3, 7.1).
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dose Modification for Use with P-gp Inhibitors
2.3 Dose Modification for Use with Combined P-gp and Strong CYP3A Inducers
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 QT/QTc Interval Prolongation
5.2 Hypersensitivity Reactions
5.3 Embryo-Fetal Toxicity
5.4 Laboratory Testing
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on ORGOVYX
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Initiate treatment of ORGOVYX with a loading dose of 360 mg on the first day and continue treatment with a 120 mg dose taken orally once daily at approximately the same time each day.
ORGOVYX can be taken with or without food [see Clinical Pharmacology (12.3)]. Instruct patients to swallow tablets whole and not to crush or chew tablets.
Advise patients to take a missed dose of ORGOVYX as soon as they remember. If the dose was missed by more than 12 hours, patients should not take the missed dose and resume with the next scheduled dose.
If treatment with ORGOVYX is interrupted for greater than 7 days, restart ORGOVYX with a loading dose of 360 mg on the first day, and continue with a dose of 120 mg once daily.
In patients treated with GnRH receptor agonists and antagonists for prostate cancer, treatment is usually continued upon development of nonmetastatic or metastatic castration-resistant prostate cancer.
2.2 Dose Modification for Use with P-gp Inhibitors
Avoid co-administration of ORGOVYX with oral P-gp inhibitors. If co-administration is unavoidable, take ORGOVYX first and separate dosing by at least 6 hours [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]. Treatment with ORGOVYX may be interrupted for up to two weeks if a short course of treatment with a P-gp inhibitor is required.
2.3 Dose Modification for Use with Combined P-gp and Strong CYP3A Inducers
Avoid co-administration of ORGOVYX with combined P-gp and strong CYP3A inducers. If co-administration is unavoidable, increase the ORGOVYX dose to 240 mg once daily. After discontinuation of the combined P-gp and strong CYP3A inducer, resume the recommended ORGOVYX dose of 120 mg once daily [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 QT/QTc Interval Prolongation
Androgen deprivation therapy, such as ORGOVYX may prolong the QT/QTc interval. Providers should consider whether the benefits of androgen deprivation therapy outweigh the potential risks in patients with congenital long QT syndrome, congestive heart failure, or frequent electrolyte abnormalities and in patients taking drugs known to prolong the QT interval. Electrolyte abnormalities should be corrected. Consider periodic monitoring of electrocardiograms and electrolytes [see Clinical Pharmacology (12.2)].
5.2 Hypersensitivity Reactions
ORGOVYX is contraindicated in patients with severe hypersensitivity to relugolix or any of the product components [see Contraindications (4)]. Hypersensitivity reactions, including pharyngeal edema and other serious cases of angioedema, have been reported in postmarketing in patients treated with ORGOVYX.
In the HERO study, patients treated with relugolix reported angioedema (0.2%) [see Clinical Trials Experience (6.1)].
Advise patients who experience any symptoms of hypersensitivity to temporarily discontinue ORGOVYX and promptly seek medical care.
Discontinue ORGOVYX for severe hypersensitivity reactions and manage as clinically indicated.
5.3 Embryo-Fetal Toxicity
The safety and efficacy of ORGOVYX have not been established in females. Based on findings in animals and mechanism of action, ORGOVYX can cause fetal harm and loss of pregnancy when administered to a pregnant female. In an animal reproduction study, oral administration of relugolix to pregnant rabbits during the period of organogenesis caused embryo-fetal lethality at maternal exposures that were 0.3 times the human exposure at the recommended dose of 120 mg daily based on area under the curve (AUC). Advise males with female partners of reproductive potential to use effective contraception during treatment and for 2 weeks after the last dose of ORGOVYX [see Use in Specific Populations (8.1, 8.3) and Clinical Pharmacology (12.1)].
5.4 Laboratory Testing
Therapy with ORGOVYX results in suppression of the pituitary gonadal system. Results of diagnostic tests of the pituitary gonadotropic and gonadal functions conducted during and after ORGOVYX may be affected. The therapeutic effect of ORGOVYX should be monitored by measuring serum concentrations of prostate specific antigen (PSA) periodically. If PSA increases, serum concentrations of testosterone should be measured.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- QT/QTc Interval Prolongation [see Warnings and Precautions (5.1)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of ORGOVYX was evaluated in HERO, a randomized (2:1), open-label, clinical study in patients with advanced prostate cancer [see Clinical Studies (14)]. Patients received orally administered ORGOVYX as a loading dose of 360 mg on the first day followed by 120 mg taken orally once daily (n = 622) or received leuprolide acetate administered by depot injection at doses of 22.5 mg (n = 264) or 11.25 mg (n = 44) per local guidelines every 12 weeks (n = 308). Leuprolide acetate 11.25 mg is a dosage regimen that is not recommended for this indication in the US. Among patients who received ORGOVYX, 91% were exposed for at least 48 weeks. Ninety-nine (16%) patients received concomitant radiotherapy and 17 (3%) patients received concomitant enzalutamide with ORGOVYX.
Serious adverse reactions occurred in 12% of patients receiving ORGOVYX. Serious adverse reactions in ≥ 0.5% of patients included myocardial infarction (0.8%), acute kidney injury (0.6%), arrhythmia (0.6%), hemorrhage (0.6%), and urinary tract infection (0.5%). Fatal adverse reactions occurred in 0.8% of patients receiving ORGOVYX including metastatic lung cancer (0.3%), myocardial infarction (0.3%), and acute kidney injury (0.2%). Fatal and non-fatal myocardial infarction and stroke were reported in 2.7% of patients receiving ORGOVYX.
Permanent discontinuation of ORGOVYX due to an adverse reaction occurred in 3.5% of patients. Adverse reactions which resulted in permanent discontinuation of ORGOVYX in ≥ 0.3 % of patients included atrioventricular block (0.3%), cardiac failure (0.3%), hemorrhage (0.3%), increased transaminases (0.3%), abdominal pain (0.3%), and pneumonia (0.3%).
Dosage interruptions of ORGOVYX due to an adverse reaction occurred in 2.7% of patients. Adverse reactions which required dosage interruption in ≥ 0.3% of patients included fracture (0.3%).
The most common adverse reactions (≥ 10%) and laboratory abnormalities (≥ 15%), were hot flush (54%), glucose increased (44%), triglycerides increased (35%), musculoskeletal pain (30%), hemoglobin decreased (28%), alanine aminotransferase increased (ALT) (27%), fatigue (26%), aspartate aminotransferase increased (AST) (18%), constipation (12%), and diarrhea (12%).
Table 1 summarizes the adverse reactions in HERO.
Table 1: Adverse Reactions ( ≥ 10%) of Patients with Advanced Prostate Cancer Who Received ORGOVYX in HERO a Includes arthralgia, back pain, pain in extremity, musculoskeletal pain, myalgia, bone pain, neck pain, arthritis, musculoskeletal stiffness, non-cardiac chest pain, musculoskeletal chest pain, spinal pain, and musculoskeletal discomfort.
b Includes fatigue and asthenia.
c Includes diarrhea and colitis.
Adverse Reaction ORGOVYX
N = 622Leuprolide Acetate
N = 308All Grades
(%)Grade 3-4
(%)All Grades
(%)Grade 3-4
(%)Vascular disorders Hot flush 54 0.6 52 0 Musculoskeletal and connective tissue disorders Musculoskeletal paina 30 1.1 29 1.6 General Fatigueb 26 0.3 24 0 Gastrointestinal disorders Diarrheac 12 0.2 7 0 Constipation 12 0 10 0 Clinically relevant adverse reactions in < 10% of patients who received ORGOVYX included increased weight, insomnia, gynecomastia, hyperhidrosis, depression, decreased libido, and angioedema.
Table 2 summarizes the laboratory abnormalities in HERO.
Table 2: Select Laboratory Abnormalities ( ≥ 15%) That Worsened from Baseline in Patients with Advanced Prostate Cancer Who Received ORGOVYX in HERO Laboratory Test ORGOVYXa Leuprolide Acetatea All Grades
(%)Grade 3-4
(%)All Grades
(%)Grade 3-4
(%)a The denominator used to calculate the rate varied from 611 to 619 in the ORGOVYX arm and from 301 to 306 in the leuprolide arm based on the number of patients with a baseline value and at least one post-treatment value.
Chemistry Glucose increased 44 2.9 54 6 Triglycerides increased 35 2 36 0.7 ALT increased 27 0.3 28 0 AST increased 18 0 19 0.3 Hematology Hemoglobin decreased 28 0.5 29 0.7 6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ORGOVYX. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune system disorders: hypersensitivity, including angioedema and urticaria.
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on ORGOVYX
P-gp Inhibitors
Relugolix is a P-gp substrate. Co-administration of ORGOVYX with an oral P-gp inhibitor increases relugolix exposure [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions associated with ORGOVYX.
Avoid co-administration of ORGOVYX with oral P-gp inhibitors.
If co-administration with an oral P-gP inhibitor cannot be avoided, take ORGOVYX first and wait at least 6 hours before taking the P-gp inhibitor. Closely monitor patients for increased adverse reactions [see Dosage and Administration (2.2)].
If an oral P-gp inhibitor will only be administered for up to 2 weeks, ORGOVYX may be interrupted while the P-gp inhibitor is being administered. Resume ORGOVYX after the P-gp is discontinued. If ORGOVYX is interrupted for more than 7 days, resume ORGOVYX at 360 mg on the first day, followed by 120 mg once daily.
Combined P-gp and Strong CYP3A Inducers
Co-administration of ORGOVYX with a combined P-gp and strong CYP3A inducer decreases relugolix exposure, which may reduce the effects of ORGOVYX [see Clinical Pharmacology (12.3)].
Avoid co-administration of ORGOVYX with combined P-gp and strong CYP3A inducers.
If co-administration cannot be avoided, increase the ORGOVYX dose. After discontinuation of the combined P-gp and strong CYP3A inducer, resume ORGOVYX once daily at the same dose [see Dosage and Administration (2.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The safety and efficacy of ORGOVYX have not been established in females.
Based on findings in animals and mechanism of action, ORGOVYX can cause fetal harm and loss of pregnancy when administered to a pregnant female [see Clinical Pharmacology (12.1)]. There are no human data on the use of ORGOVYX in pregnant females to inform the drug-associated risk. In an animal reproduction study, oral administration of relugolix to pregnant rabbits during organogenesis caused embryo-fetal lethality at maternal exposures that were 0.3 times the human exposure at the recommended dose of 120 mg daily based on AUC (see Data). Advise patients of the potential risk to the fetus.
Data
Animal Data
In an embryo-fetal development study, oral administration of relugolix to pregnant rabbits during the period of organogenesis resulted in abortion, total litter loss, or decreased number of live fetuses at a dose of 9 mg/kg/day (approximately 0.3 times the human exposure at the recommended dose of 120 mg daily based on AUC).
8.2 Lactation
Risk Summary
The safety and efficacy of ORGOVYX at the recommended dose of 120 mg daily have not been established in females. There are no data on the presence of relugolix in human milk, the effects on the breastfed child, or the effects on milk production. Relugolix and/or its metabolites were present in milk of lactating rats (see Data).
8.3 Females and Males of Reproductive Potential
Males
Based on findings in animals and mechanism of action, advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 2 weeks after the last dose of ORGOVYX [see Use in Specific Populations (8.1)].
8.4 Pediatric Use
The safety and efficacy of ORGOVYX in pediatric patients have not been established.
8.5 Geriatric Use
Of the 622 patients who received ORGOVYX in the HERO study, 81% were 65 years of age or older, while 35% were 75 years of age or older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. There was no clinically relevant impact of age on the pharmacokinetics of ORGOVYX or testosterone response based on population pharmacokinetic and pharmacokinetic/pharmacodynamic analyses in men 45 to 91 years of age.
-
11 DESCRIPTION
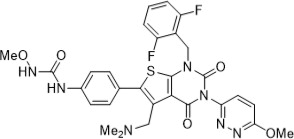
Relugolix is a nonpeptide small molecule, GnRH receptor antagonist. The chemical name is N-(4-{1-[(2,6-difluorophenyl)methyl]-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl}phenyl)-N'-methoxyurea.
The molecular weight is 623.63 daltons and the molecular formula is C29H27F2N7O5S. The structural formula is:
Relugolix is a white to off-white to slightly yellow solid with a solubility of 0.04 mg per mL in water at 25°C.
ORGOVYX is provided as film-coated tablets for oral administration. Each tablet contains 120 mg of relugolix. The inactive ingredients are mannitol, sodium starch glycolate, hydroxypropyl cellulose, magnesium stearate, hypromellose, titanium dioxide, ferric oxide red, and carnauba wax.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Relugolix is a nonpeptide GnRH receptor antagonist that competitively binds to pituitary GnRH receptors, thereby, reducing the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and consequently testosterone.
12.2 Pharmacodynamics
Pituitary and Gonadal Hormones
Relugolix reduced LH, FSH (Figure 1), and testosterone concentrations after oral administration of the recommended loading dose of 360 mg and a 120 mg dose once daily.
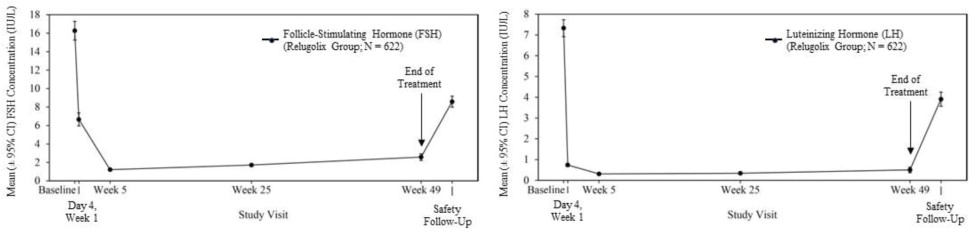
Out of 622 patients, 56% had testosterone concentrations at castrate levels (< 50 ng/dL) by the first sampling timepoint at Day 4, and 97% maintained castrate levels of testosterone through 48 weeks. In a substudy of 137 patients who did not receive subsequent androgen deprivation therapy for at least 90 days after discontinuation of relugolix, the cumulative incidence rate of achieving testosterone concentrations above the lower limit of the normal range (> 280 ng/dL) or baseline at 90 days was 55% [see Clinical Studies (14)].
Figure 1: Mean (± 95% CI) Follicle-Stimulating Hormone and Luteinizing Hormone Concentrations over Time in HERO
Cardiac Electrophysiology
In a randomized, double-blind, placebo- and positive-controlled (open-label moxifloxacin), parallel-group thorough QT/QTc study, no increase in mean QTc interval > 10 ms was identified after administration of a single 60 mg or 360 mg (0.2 or 1 times the recommended loading dose, respectively) relugolix dose.
12.3 Pharmacokinetics
After administration of single doses ranging from 60 mg to 360 mg (0.17 to 1 times the recommended loading dose), the area under concentration-time curve from time zero to infinity (AUC0-inf) and the maximum observed plasma concentration (Cmax) of relugolix increased approximately proportionally with dose. After administration of multiple doses of relugolix once daily, the AUCtau of relugolix increased approximately proportionally with dose while the Cmax increase was greater than dose proportional for doses from 20 mg to 180 mg (0.17 to 1.5 times the recommended dosage). After administration of a single 360 mg loading dose in patients, the mean (± standard deviation [± SD]) of AUC0-24 and Cmax of relugolix were 985 (± 742) ng.hr/mL and 215 (± 184) ng/mL, respectively. After administration of a 120 mg dose once daily in patients, the mean (± SD) of AUC0-24 and Cmax of relugolix at steady-state were 407 (± 168) ng.hr/mL and 70 (± 65) ng/mL, respectively. The accumulation of relugolix upon once daily administration is approximately 2-fold.
Absorption
Relugolix is a substrate for intestinal P-gp. The mean (CV%) absolute bioavailability of relugolix is 12% (62%). The median (range) Tmax of relugolix is 2.25 hours (0.5 to 5.0 hours).
Effect of Food
No clinically meaningful differences in the pharmacokinetics of relugolix were observed following consumption of a high-calorie, high-fat meal (approximately 800 to 1000 calories with 500, 220, and 124 from fat, carbohydrate, and protein, respectively).
Distribution
Plasma protein binding of relugolix is 68 to 71%, primarily to albumin and to a lesser extent to α1-acid glycoprotein. The mean blood-to-plasma ratio is 0.78.
Specific Populations
No clinically meaningful differences in the pharmacokinetics of relugolix were observed based on age (45 to 91 years), race/ethnicity (Asian [19%], White [71%], Black/African American [6%]), body weight (41 to 193 kg), mild to severe renal impairment (creatinine clearance [CLcr] 15 to 89 mL/min, as estimated by the Cockcroft-Gault equation), or mild to moderate hepatic impairment (Child-Pugh A or B). The effect of end-stage renal disease with or without hemodialysis or severe hepatic impairment (Child-Pugh C) on the pharmacokinetics of relugolix has not been evaluated.
Drug Interactions Studies
Clinical Studies
Combined P-gp and Moderate CYP3A Inhibitors: Co-administration with erythromycin (P-gp and moderate CYP3A inhibitor) increased the AUC and Cmax of relugolix by 3.5- and 2.9-fold respectively.
Combined P-gp and Strong CYP3A Inducers: Co-administration of relugolix with rifampin (P-gp and strong CYP3A inducer) decreased the AUC and Cmax of relugolix by 55% and 23%, respectively.
Other Drugs: No clinically significant differences in the pharmacokinetics of relugolix were observed when co-administered with voriconazole (strong CYP3A inhibitor), atorvastatin, enzalutamide, or acid-reducing agents. No clinically significant differences in the pharmacokinetics of midazolam (sensitive CYP3A substrate) or rosuvastatin (BCRP substrate), or dabigatran etexilate (P-gp substrate) were observed upon co-administration with relugolix.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies were conducted in mice at oral relugolix doses up to 100 mg/kg/day and in rats at doses up to 600 mg/kg/day. Relugolix was not carcinogenic in mice or rats at exposures up to approximately 75 or 224 times, respectively, the human exposure at the recommended dose of 120 mg daily based on AUC.
Relugolix was not mutagenic in the in vitro bacterial reverse mutation (Ames) assay or clastogenic in the in vitro chromosomal aberration assay in Chinese hamster lung cells or the in vivo rat bone marrow micronucleus assay.
In human GnRH-receptor knock-in male mice, oral administration of relugolix decreased prostate and seminal vesicle weights at doses ≥ 3 mg/kg twice daily for 28 days. The effects of relugolix were reversible, except for testis weight, which did not fully recover within 28 days after drug withdrawal. In a 39-week repeat-dose toxicity study in monkeys, there were no significant effects on male reproductive organs at oral relugolix doses up to 50 mg/kg/day (approximately 53 times the human exposure at the recommended dose of 120 mg daily based on AUC).
13.2 Animal Toxicology and/or Pharmacology
Phospholipidosis (intracellular phospholipid accumulation) was observed in multiple organs and tissues (e.g., liver, pancreas, spleen, kidney, lymph nodes, lung, bone marrow, gastrointestinal tract or testes) after repeated oral administration of relugolix in rats and monkeys. In a rat 26-week toxicity study, phospholipidosis was observed at doses ≥ 100 mg/kg (approximately 18 times the human exposure at the recommended dose based on AUC). In a monkey 39-week toxicity study, this effect was observed at doses ≥ 1.5 mg/kg (approximately 0.6 times the human exposure at the recommended dose based on AUC) and demonstrated evidence of reversibility after cessation of treatment. The significance of this finding in humans is unknown.
-
14 CLINICAL STUDIES
HERO Study
The safety and efficacy of ORGOVYX was evaluated in HERO (NCT03085095), a randomized, open label study in men with advanced prostate cancer requiring at least 1 year of androgen deprivation therapy and defined as biochemical (PSA) or clinical relapse following local primary intervention, newly diagnosed castration-sensitive metastatic disease, or advanced localized disease.
A total of 934 patients were randomized to receive ORGOVYX or leuprolide in a 2:1 ratio for 48 weeks:
- a)
- ORGOVYX at a loading dose of 360 mg on the first day followed by daily doses of 120 mg orally.
- b)
- Leuprolide acetate 22.5 mg injection (or 11.25 mg in Japan and Taiwan) subcutaneously every 3 months. Leuprolide acetate 11.25 mg is a dosage regimen that is not recommended for this indication in the US.
Serum testosterone concentrations were measured at screening; on Days 1, 4, 8, 15, and 29 in the first month; then monthly until the end of the study.
The population (N = 930) across both treatment groups had a median age of 71 years (range 47 to 97 years). The ethnic/racial distribution was 68% White, 21% Asian, 4.9% Black, and 5% other. Disease stage was distributed as follows: 32% metastatic (M1), 31% locally advanced (T3/4 NX M0 or any T N1 M0), 28% localized (T1 or T2 N0 M0), and 10% not classifiable. The median testosterone concentration at baseline across the treatment groups was 408 ng/dL.
The major efficacy outcome measure was medical castration rate defined as achieving and maintaining serum testosterone suppression to castrate levels (< 50 ng/dL) by Day 29 through 48 weeks of treatment. Other endpoints included castration rates on Day 4 and 15 and castration rates with testosterone < 20 ng/dL at Day 15.
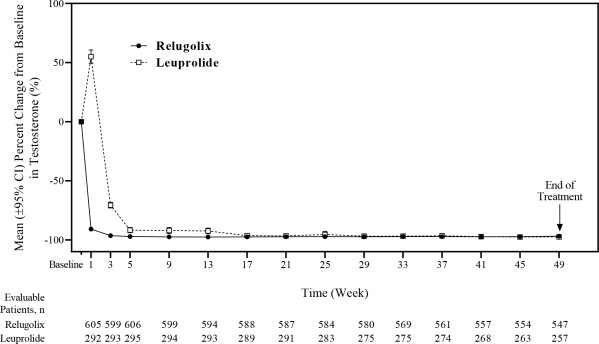
The efficacy results are shown in Table 3 and the time course of percent change from baseline in testosterone suppression by ORGOVYX and leuprolide during the 48 week treatment period are shown in Figure 2.
Table 3: Medical Castration Rates (Testosterone Concentrations < 50 ng/dL) from Day 29 through Week 48 in HERO a 11.25 mg is a dosage regimen that is not recommended for this indication in the US. The castration rate of the subgroup of patients receiving 22.5 mg leuprolide (n = 264) was 88.0% (95% CI: 83.4%, 91.4%).
b Two patients in each arm did not receive the study treatment and were not included.
c Kaplan-Meier estimates within group.
ORGOVYX
360/120 mg
(N = 622)bLeuprolide Acetate
22.5 or 11.25 mga
(N = 308)bCastration Rate (95% CI)c 96.7%
(94.9%, 97.9%)88.8%
(84.6%, 91.8%)Figure 2: Mean (95% CI) Percent Change from Baseline in Testosterone Concentrations from Baseline to Week 49 by Treatment Group in HERO
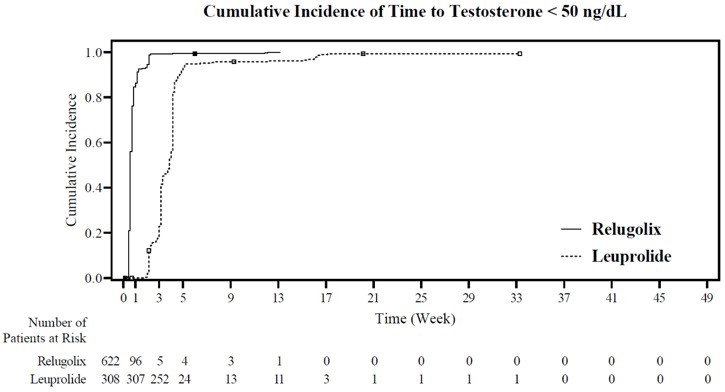
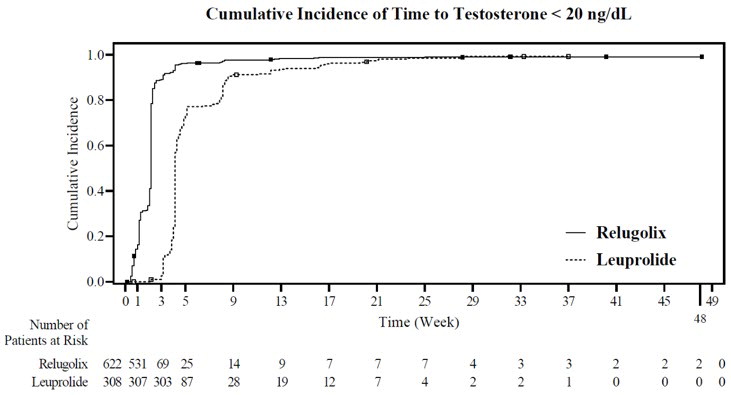
The percentages of patients who attained the medical castration levels of testosterone < 50 ng/dL and < 20 ng/dL within the first 29 days of treatment are summarized in Table 4 and the cumulative incidences of time to testosterone < 50 ng/dL or < 20 ng/dL are shown in Figure 3.
Table 4: Percentage of Patients Attaining Testosterone Decreases within the First 29 Days in HEROa Testosterone < 50 ng/dL Testosterone < 20 ng/dL ORGOVYX
(N = 622)Leuprolide Acetate
(N = 308)ORGOVYX
(N = 622)Leuprolide Acetate
(N = 308)a Kaplan-Meier estimates within group.
Day 4 56% 0% 7% 0% Day 8 91% 0% 27% 0% Day 15 99% 12% 78% 1% Day 29 99% 82% 95% 57% Figure 3: Cumulative Incidence of Time to Testosterone < 50 ng/dL and < 20 ng/dL in HERO
In the clinical trial, PSA levels were monitored and were lowered on average by 65% two weeks after administration of ORGOVYX, 83% after 4 weeks, 92% after 3 months and remained suppressed throughout the 48 weeks of treatment. These PSA results should be interpreted with caution because of the heterogeneity of the patient population studied. No evidence has shown that the rapidity of PSA decline is related to a clinical benefit.
A substudy was conducted in 137 patients who did not receive subsequent androgen deprivation therapy for at least 90 days after discontinuation of ORGOVYX. Based on Kaplan-Meier analyses, 55% of patients achieved testosterone levels above the lower limit of the normal range (> 280 ng/dL) or baseline at 90 days after discontinuation of ORGOVYX.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
The 120 mg tablets are film-coated, light red, almond shaped, and debossed with “R” on one side and “120” on the other side and are supplied in two configurations, bottles and blister packs. Each bottle (NDC 72974-120-01) contains 30 tablets and a desiccant and is closed with a child resistant induction seal cap. The blister cards contain nine tablets packaged in a carton (NDC 72974-120-02). Each ORGOVYX tablet contains 120 mg of relugolix.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
QT/QTc Interval Prolongation
- Advise patients that androgen deprivation therapy treatment with ORGOVYX may prolong the QT interval. Inform patients of the signs and symptoms of QT prolongation. Advise patients to contact their healthcare provider immediately for signs or symptoms of QT prolongation [see Warnings and Precautions (5.1)].
Hypersensitivity
- Inform patients that if they have experienced severe hypersensitivity with relugolix or to any of the product components, ORGOVYX is contraindicated [see Contraindications (4)].
- Inform patients that ORGOVYX can cause severe hypersensitivity reactions that include angioedema [see Warnings and Precautions (5.2)].
- Advise patients who experience hypersensitivity symptoms to discontinue ORGOVYX and promptly contact their healthcare provider.
Androgen Deprivation
- Inform patients about adverse reactions related to androgen deprivation therapy with ORGOVYX, including hot flashes, flushing of the skin, increased weight, decreased sex drive, and difficulties with erectile function [see Adverse Reactions (6.1)].
Embryo-Fetal Toxicity
- Inform patients that ORGOVYX can be harmful to a developing fetus and can cause loss of pregnancy.
- Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 2 weeks after the last dose of ORGOVYX [see Warnings and Precautions (5.3) and Use in Specific Populations (8.1, 8.3)].
Infertility
- Inform patients that ORGOVYX may cause infertility [see Use in Specific Populations (8.3)].
Drug Disposal
- Advise patients to dispose of unused medication via a take-back option if available. Otherwise, advise to follow FDA instructions for disposing medication in the household trash, www.fda.gov/drugdisposal and NOT to flush down the toilet.
Manufactured for Sumitomo Pharma America, Inc., Marlborough, MA 01752
214621-MS-005
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 08/2023
PATIENT INFORMATION
ORGOVYX (or-GO-vix)
(relugolix)
TabletsWhat is ORGOVYX?
ORGOVYX is a prescription medicine used in adults for the treatment of advanced prostate cancer.
It is not known if ORGOVYX is safe or effective in females.
It is not known if ORGOVYX is safe or effective in children.Do not take ORGOVYX if you have had a severe allergic reaction to relugolix or any of the ingredients in ORGOVYX. See the end of this Patient Information for a complete list of the ingredients in ORGOVYX. Before taking ORGOVYX, tell your healthcare provider about all of your medical conditions, including if you:
- have any heart problems, including a condition called long QT syndrome.
- are pregnant or plan to become pregnant. ORGOVYX can harm your unborn baby and cause loss of pregnancy (miscarriage).
- have a partner who is pregnant or may become pregnant.
- Males who have female partners who are able to become pregnant should use effective birth control (contraception) during treatment with ORGOVYX and for 2 weeks after the last dose of ORGOVYX.
- are breastfeeding or plan to breastfeed. It is not known if ORGOVYX passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking ORGOVYX with certain other medicines can affect how ORGOVYX works or may cause side effects.
You should not start or stop any medicine before you talk with your healthcare provider who prescribed ORGOVYX.
Know the medicines you take. Keep a list of them with you to show to your healthcare provider and pharmacist when you get a new medicine.How should I take ORGOVYX?
- Take ORGOVYX exactly as your healthcare provider tells you.
- Take 3 ORGOVYX tablets on your first day of treatment. After that, take 1 ORGOVYX tablet each day.
- Take ORGOVYX at about the same time each day.
- Take ORGOVYX with or without food.
- Swallow ORGOVYX tablets whole. Do not crush or chew tablets.
- Your healthcare provider may change your dose if needed.
- Do not change your dose or stop taking ORGOVYX without talking with your healthcare provider first.
- If you miss a dose of ORGOVYX, take it as soon as you remember. If the dose was missed by more than 12 hours, the missed dose should not be taken. Take your next dose at your regular time the next day.
What are the possible side effects of ORGOVYX?
ORGOVYX may cause serious side effects, including:
-
Changes in the electrical activity of your heart (QT prolongation). Your healthcare provider may check your body salts (electrolytes) and the electrical activity of your heart during treatment with ORGOVYX. Tell your healthcare provider right away if you get any signs or symptoms of QT prolongation, including:
- dizziness
- fainting
- feeling that your heart is pounding or racing (palpitations)
- chest pain
-
Allergic reactions. Stop taking ORGOVYX and tell your healthcare provider or get emergency medical help right away if you get any signs or symptoms of an allergic reaction, including:
- swelling of your face, lips, tongue, throat, or trouble swallowing
- trouble breathing
- hives (raised bumps), rash, or redness all over your body
The most common side effects of ORGOVYX include:
- hot flushes
- increased blood sugar levels
- increased blood fat (triglyceride) levels
- muscle and joint pain
- decreased blood hemoglobin levels
- increased liver enzymes
- tiredness
- constipation
- diarrhea
Other side effects include weight gain, decreased sex drive, and erectile function problems.
ORGOVYX may cause fertility problems in males, which may affect your ability to father children. Talk to your healthcare provider if this is a concern for you.
These are not all the possible side effects of ORGOVYX.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store ORGOVYX?
- Store ORGOVYX at room temperature. Do not store ORGOVYX above 86°F (30°C).
- Keep the bottle tightly closed after you first open it.
- The ORGOVYX bottle contains a desiccant to help keep your medicine dry (protect it from moisture). Do not remove the desiccant from the bottle.
- Dispose of unused medicines through community take-back disposal programs when available. If no community take-back disposal program is available go to www.fda.gov/drugdisposal for information on how to dispose of ORGOVYX the right way.
- Do not flush ORGOVYX down the toilet.
Keep ORGOVYX and all medicines out of the reach of children. General information about the safe and effective use of ORGOVYX.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ORGOVYX for a condition for which it was not prescribed. Do not give ORGOVYX to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ORGOVYX that is written for health professionals.What are the ingredients in ORGOVYX?
Active ingredient: relugolix
Inactive ingredients: mannitol, sodium starch glycolate, hydroxypropyl cellulose, magnesium stearate, hypromellose, titanium dioxide, ferric oxide red, and carnauba wax.
Manufactured for: Sumitomo Pharma America, Inc., Marlborough, MA 01752
For more information, go to www.orgovyx.com or call 1-833-696-8268.214621-MS-005
- PRINCIPAL DISPLAY PANEL - 120 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
ORGOVYX
relugolix tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72974-120 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength relugolix (UNII: P76B05O5V6) (relugolix - UNII:P76B05O5V6) relugolix 120 mg Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) HYDROXYPROPYL CELLULOSE (90000 WAMW) (UNII: UKE75GEA7F) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color RED (light red) Score no score Shape FREEFORM (almond) Size 11mm Flavor Imprint Code R;120 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72974-120-01 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/18/2020 2 NDC:72974-120-97 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/21/2021 3 NDC:72974-120-95 16 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/27/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214621 12/18/2020 Labeler - Sumitomo Pharma America, Inc. (131661746) Registrant - Sumitomo Pharma Switzerland GmbH (480146015)