Label: DISPOSABLE CONVENIENCE KIT (SINGLE SHOT EPIDURAL)- lidocaine hydrochloride, sodium chloride, proidone iodine, bupivacaine kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 69938-153-21 - Packager: True Fit RX LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated February 9, 2016
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
BUPIVACAINE HYDROCHLORIDE injection, solution
BUPIVACAINE HYDROCHLORIDE AND EPINEPHRINE (bupivacaine hydrochloride and epinephrine bitartrate) injection, solution
[Hospira, Inc.]Rx only
THE 0.75% CONCENTRATION OF BUPIVACAINE HYDROCHLORIDE IS NOT RECOMMENDED FOR OBSTETRICAL ANESTHESIA.
THERE HAVE BEEN REPORTS OF CARDIAC ARREST WITH DIFFICULT RESUSCITATION OR DEATH DURING USE OF BUPIVACAINE HYDROCHLORIDE FOR EPIDURAL ANESTHESIA IN OBSTETRICAL PATIENTS. IN MOST CASES, THIS HAS FOLLOWED USE OF THE 0.75% CONCENTRATION. RESUSCITATION HAS BEEN DIFFICULT OR IMPOSSIBLE DESPITE APPARENTLY ADEQUATE PREPARATION AND APPROPRIATE MANAGEMENT. CARDIAC ARREST HAS OCCURRED AFTER CONVULSIONS RESULTING FROM SYSTEMIC TOXICITY, PRESUMABLY FOLLOWING UNINTENTIONAL INTRAVASCULAR INJECTION. THE 0.75% CONCENTRATION SHOULD BE RESERVED FOR SURGICAL PROCEDURES WHERE A HIGH DEGREE OF MUSCLE RELAXATION AND PROLONGED EFFECT ARE NECESSARY.
DESCRIPTION
Bupivacaine Hydrochloride is 2-Piperidinecarboxamide, 1-butyl-N-(2,6-dimethylphenyl)-, monohydrochloride, monohydrate, a white crystalline powder that is freely soluble in 95 percent ethanol, soluble in water, and slightly soluble in chloroform or acetone. It has the following structural formula:
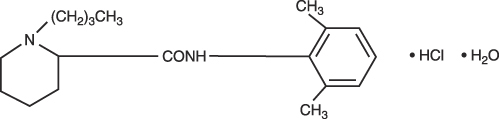
bupivacaine hydrochloride 1
Epinephrine is (-)-3,4-Dihydroxy-α-[(methylamino)methyl] benzyl alcohol. It has the following structural formula:
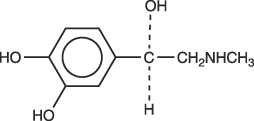
bupivacaine hydrochloride 2
infiltration, peripheral nerve block, and caudal and lumbar epidural blocks. Solutions of Bupivacaine Hydrochloride may be autoclaved if they do not contain epinephrine. Solutions are clear and colorless.
Bupivacaine is related chemically and pharmacologically to the aminoacyl local anesthetics. It is a homologue of mepivacaine and is chemically related to lidocaine. All three of these anesthetics contain an amide linkage between the aromatic nucleus and the amino, or piperidine group. They differ in this respect from the procaine-type local anesthetics, which have an ester linkage.
Bupivacaine Hydrochloride Injection, USP is available in sterile, isotonic solutions containing bupivacaine hydrochloride in water for injection with characteristics as follows:
Buivacaine Hydrochloride Injection USP (without epinephrine) Concentration Bupivacaine Hydrochloride mg/ml Sodium Chloride mg/ml 0.25% 2.5 8.6 0.5% 5 8.1 0.75% 7.5 7.6 May contain sodium hydroxide and/or hydrochloric acid for pH adjustment. (See HOW SUPPLIED section for pH information.) Multiple-dose vials contain methylparaben 1 mg/mL added as a preservative.
Bupivacaine and Epinephrine Injection, USP is available in sterile, isotonic solutions containing bupivacaine hydrochloride and epinephrine 1:200,000 with characteristics as follows:
Bupivacaine and Epinephrine Injection, USP Concentration (Bupivacaine HCL) Bupivacaine Hydrochloride (mg/ml) Epinephrine 1:2000,000 (mcg/ml) Sodium Chloride (mg/ml) 0.25% 2.5 5 8.5 0.5% 5 5 8.5 0.75% 7 Sodium metabisulfite 0.1 mg/mL added as antioxidant and edetate calcium disodium, anhydrous 0.1 mg/mL added as stabilizer. May contain sodium hydroxide and/or hydrochloric acid for pH adjustment. (See HOW SUPPLIED section for pH information.) Multiple-dose vials contain methylparaben 1 mg/mL added as a preservative.
Single-dose solutions contain no added bacteriostat or anti-microbial agent and unused portions should be discarded after use.
-
DESCRIPTION
APLICARE POVIDONE-IODINE SOLUTION (povidone-iodine solution) solution
3/4 FLUID OUNCEPovidone-iodine 10%
AntisepticWarnings
Do not use:
- if allergic to iodine
- in the eyes
For external use only
Ask a doctor before use if injuries are
- deep or puncture wounds
- serious burns
Stop use and ask a doctor if
- redness, irritation, swelling or pain persists or
- increases infection occurs
Avoid pooling beneath patient
Keep out of reach of children.
In case of accidental ingestion, seek professional assistance or consult a poison control center immediately.

Aplicare Povidone Iodine PDP
-
DESCRIPTION
LIDOCAINE HYDROCHLORIDE (lidocaine hydrochloride anhydrous) injection, solution
AQUEOUS SOLUTIONS FOR INFILTRATION
AND NERVE BLOCK
Ampul
Plastic Multiple-dose Fliptop Vial
Glass Teartop Vial
Rx only
DESCRIPTION
Lidocaine Hydrochloride Injection, USP is a sterile, nonpyrogenic solution of lidocaine hydrochloride in water for injection for parenteral administration in various concentrations with characteristics as follows:Concentration 0.5% 1% 1.5% 2% mg/ml lidocaine HCL (anhyd.) 5 10 15 20 mg/ml sodium chloride 8 7 6 Multiple-dose vials contain 0.1% of methylparaben added as preservative. May contain sodium hydroxide and/or hydrochloric acid for pH adjustment. The pH is 6.5 (5.0 to 7.0). See HOW SUPPLIED section for various sizes and strengths.
Lidocaine is a local anesthetic of the amide type.
Lidocaine Hydrochloride, USP is chemically designated 2-(diethylamino)-N-(2,6-dimethylphenyl)-acetamide monohydrochloride monohydrate, a white powder freely soluble in water. The molecular weight is 288.82. It has the following structural formula:
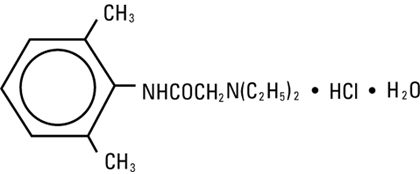
lidocaine hydrochloride injection figure
The semi-rigid vial used for the plastic vials is fabricated from a specially formulated polyolefin. It is a copolymer of ethylene and propylene. The safety of the plastic has been confirmed by tests in animals according to USP biological standards for plastic containers. The container requires no vapor barrier to maintain the proper drug concentration.

- DESCRIPTION
-
DESCRIPTION
Sodium Chloride Injection, USP is a sterile, nonpyrogenic, isotonic solution of sodium chloride 0.9% (9 mg/mL) in Water for Injection containing no antimicrobial agent or other added substance. The pH is between 4.5 and 7.0. Its chloride and sodium ion concentrates are approximately 0.154 mEq of each per milliliter and its calculated osmolality is 0.308 milliosmols per mL.
Sodium chloride occurs as colorless cubic crystals or white crystalline powder and has a saline taste.
Sodium Chloride is freely soluble in water. It is soluble in glycerin and slightly soluble in alcohol. The empirical formula for sodium chloride is NaCl, and the molecular weight is 58.44.

Sodium chloride comprises over 90% of the inorganic constituents of the blood serum. Sodium
chloride in water dissociates to provide sodium (Na+) and chloride (Cl-) ions. These ions are normal
constituents of the body fluids (principally extracellular) and are essential for maintaining electrolyte balance. The small volume of Fluid and amount of sodium chloride provided by Sodium Chloride Injection, USP, 0.9% when used only as a vehicle for parenteral injection of drugs, is unlikely to exert a significant effect on fluid and electrolyte balance except possibly in very small infants.Sodium Chloride must be used with caution in the presence of congestive heart failure, circulatory
insufficiency, kidney dysfunction or hypoproteinemia. Excessive amounts of sodium chloride by any route may cause hypokalemia and acidosis.Excessive amounts by parental routes may precipitate congestive heart failure and acute pulmonary edema, especially seen in patients with preexisting cardiovascular disease and those receiving corticos-teroids, corticotropin or other drugs that may give rise to sodium retention. For use in newborns, when a Sodium Chloride solution is required for preparation or diluting medications,
or in flushing intravenous catheters, only preservative-free Sodium Chloride Injection, USP,
0.9% should be used.Sodium Chloride Injection is used to flush intravascular catheters or as a sterile, isotonic single dose vehicle, solvent, or diluent for substances to be administered intravenously, intramuscularly or subcutaneously and for other extemporaneously prepared single dose sterile solutions according to
instructions of the manufacture of the drug to be administered.Since Sodium Chloride Injection does not contain antimicrobial agents and is intended for single use,
any unused amount must be discarded immediately following withdrawal of any portion of the contents
of the vial or ampul. Do not open ampul until it is to be used.Consult the manufactures instructions for choice of vehicle, appropriate dilution or volume for
dissolving the drug to be injected, including the route and rate of injection.Pregnancy Category C: Animal reproductive studies have not been conducted with Sodium Chloride
Injection USP 0.9%. It is also not known whether Sodium Chloride Injection USP 0.9% can cause fetal
harm when administered to a pregnant woman or can affect reproduction capacity. Sodium Chloride
Injection USP 0.9% should be given to a pregnant woman only if clearly needed.Reactions which may occur because of this solution, added drugs or the technique of reconstitution or
administration include febrile response, local tenderness, abscess, tissue necrosis or infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection and extravasation.If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate
countermeasures, and if possible, retrieve and save the remainder of the unused vehicle for examination.When used as a diluent, solvent or intravascular flushing solution, this parental preparation is unlikely to pose a threat of sodium chloride or fluid overload except possible in very small infants. In the event these should occur, reevaluate the patient and institute appropriate corrective measures.
Before Sodium Chloride Injection, USP, 0.9% is used as a vehicle for the administration of a drug,
specific references should be checked for any possible incompatibility with sodium chloride. The
volume of the preparation to be used for diluting or dissolving any drug for injection is dependent on
the vehicle concentration, dose and route of administration as recommended by the manufacture.
Sodium Chloride Injection, USP, 0.9% is also indicated for use in flushing intravenous catheters.Prior to and after administration of the medication, the intravenous catheter should be flushed in its entirety with Sodium Chloride Injection, USP, 0.9%. Use in accord with any warnings or precautions appropriate to the medication being administered. Parental drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DISPOSABLE CONVENIENCE KIT (SINGLE SHOT EPIDURAL)
lidocaine hydrochloride, sodium chloride, proidone iodine, bupivacaine kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69938-153 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69938-153-21 1 in 1 KIT; Type 1: Convenience Kit of Co-Package 02/04/2016 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 AMPULE 10 mL in 4 Part 2 1 PACKET 22.5 mL in 4 Part 3 1 AMPULE 30 mL in 4 Part 4 1 AMPULE 5 mL in 4 Part 1 of 4 SODIUM CHLORIDE
sodium chloride solution injection, solutionProduct Information Route of Administration EPIDURAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 9 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 KIT 1 10 mL in 1 AMPULE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/04/2016 Part 2 of 4 APLICARE POVIDONE IODINE
povidone iodine solution solutionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 0.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) NONOXYNOL-9 (UNII: 48Q180SH9T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 22.5 mL in 1 PACKET; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 12/10/2015 Part 3 of 4 BUPIVACAINE HYDROCHLORIDE
bupivacaine hydrochloride solutionProduct Information Route of Administration INFILTRATION, EPIDURAL, INTRACAUDAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUPIVACAINE HYDROCHLORIDE (UNII: 7TQO7W3VT8) (BUPIVACAINE - UNII:Y8335394RO) BUPIVACAINE HYDROCHLORIDE 2.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 30 mL in 1 AMPULE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA070586 02/04/2016 Part 4 of 4 LIDOCAINE HYDROCHLORIDE
lidocaine hydrochloride anhydrous injection, solutionProduct Information Route of Administration SUBCUTANEOUS, INFILTRATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM CHLORIDE (UNII: 451W47IQ8X) 7 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 5 mL in 1 AMPULE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA080408 02/04/2016 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/04/2016 Labeler - True Fit RX LLC (079868455) Registrant - True Fit RX LLC (079868455) Establishment Name Address ID/FEI Business Operations True Fit RX LLC 079868455 repack(69938-153)


