Label: BSS- balanced salt solution solution
- NDC Code(s): 0065-0795-15, 0065-0795-25, 0065-0795-30, 0065-0795-50
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated January 25, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
BSS™ Sterile Irrigating Solution is a sterile balanced salt solution, each mL containing sodium chloride (NaCl) 0.64%, potassium chloride (KCl) 0.075%, calcium chloride dihydrate (CaCl2•2H2O) 0.048%, magnesium chloride hexahydrate (MgCl2•6H2O) 0.03%, sodium acetate trihydrate (C2H3NaO2•3H2O) 0.39%, sodium citrate dihydrate (C6H5Na3O7•2H2O) 0.17%, sodium hydroxide and/or hydrochloric acid (to adjust pH), and water for injection. The pH is approximately 7.5. The osmolality is approximately 300 mOsm/Kg.
- CLINICAL PHARMACOLOGY
- INDICATIONS AND USAGE
-
WARNINGS
• NOT FOR INJECTION OR INTRAVENOUS INFUSION.
• Do not use unless product is clear, seal is intact, and container is undamaged.
• Do not use if product is discolored or contains a precipitate.
• SINGLE patient use only. The contents of this bottle should not be used in more than one patient.
• This solution contains no preservative, unused contents should be discarded.
-
PRECAUTIONS
Open under aseptic conditions only.
Prior to use, check the following: tip should be firmly in place, irrigating needle should be properly seated; squeeze out several drops before inserting into anterior chamber. The needle should be removed from the anterior chamber prior to releasing pressure to prevent suction.
Studies suggest that intraocular irrigating solutions which are iso-osmotic with normal aqueous fluids should be used with caution in diabetic patients undergoing vitrectomy since intraoperative lens changes have been observed.
There have been reports of corneal clouding or edema following ocular surgery in which BSS Sterile Irrigating Solution was used as an irrigating solution.
- ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
The adapter plug is designed to accept an irrigating needle. Tissues may be irrigated by attaching the needle to the DROP-TAINER® bottle as explained below. External irrigation may be done without the irrigating needle.
Method of using Adapter Plug for LUER-LOK® Hub Ophthalmic Irrigating Needle:
1. Aseptically remove DROP-TAINER bottle from blister by peeling paper backing.
2. Snap on surgeon's sterile irrigation needle. Push until firmly in place and twist slightly.
3. Test assembly for proper function before use.
NOTE: LUER-LOK is a registered trademark of Becton, Dickinson and Company.
-
HOW SUPPLIED
In 15 mL and 30 mL sterile DROP-TAINER bottles (consisting of a polypropylene bottle and LEUR-LOK™ adapter plug with a white polypropylene closure) in a blister package.
15 mL in a 15 mL bottle:
NDC0065-0795-1530 mL in a 30 mL bottle:
NDC0065-0795-30STORAGE:Store at 36° - 77°F (2° - 25°C).
Revised: May 2021
Alcon
Distributed by:
Alcon Laboratories , Inc.
6201 South Freeway
Fort Worth, Texas 76134 USA© 2021 Alcon Inc.
W300048945-0521 -
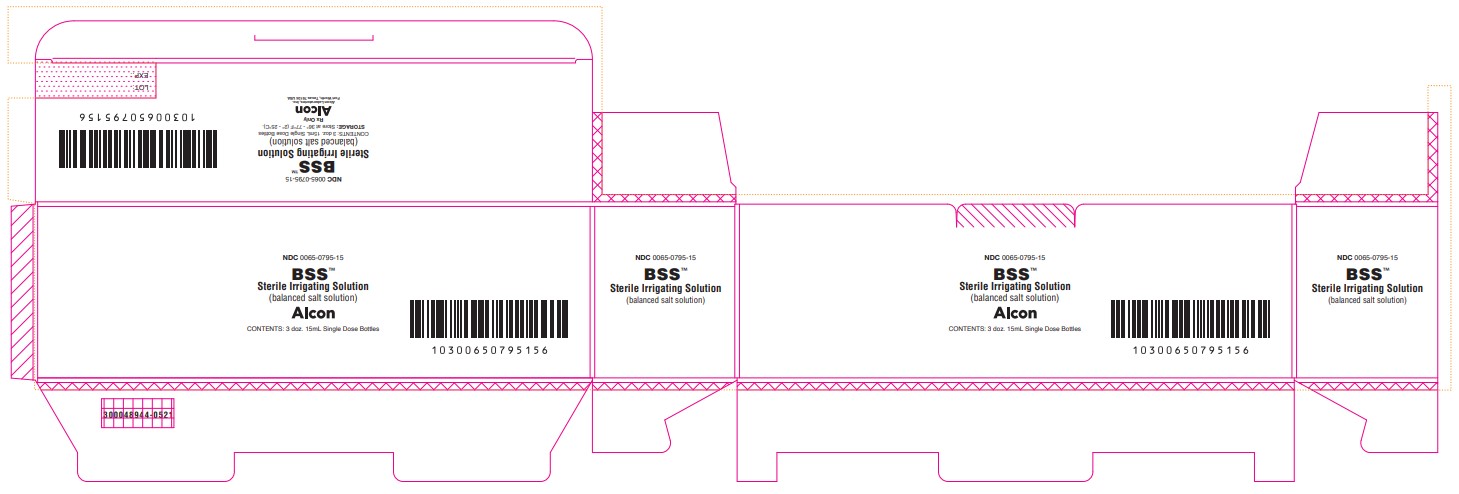
PRINCIPAL DISPLAY PANEL
NDC 0065-0795-15
BSS™
Sterile Irrigating Solution
(balanced salt solution)
ALCON
CONTENTS: 3 doz. 15mL Single Dose Bottles
STORAGE: Store at 36° - 77°F (2° - 25°C).
Rx Only
Alcon
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
300048944-0521
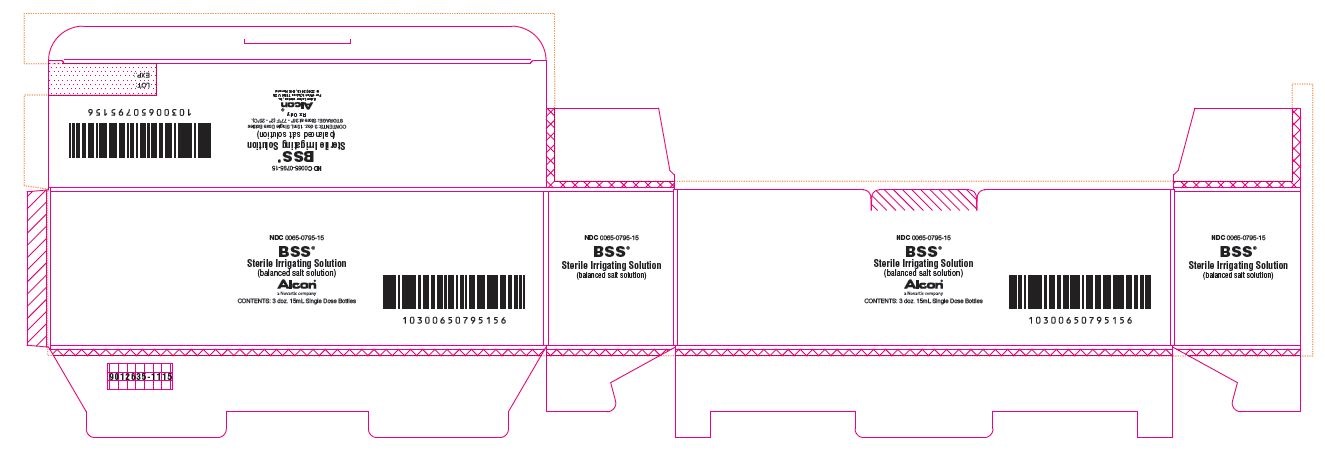
NDC 0065-0795-15
BSS®
Sterile Irrigating Solution
(balanced salt solution)
ALCON®
a Novartis company
CONTENTS: 3 doz. 15mL Single Dose Bottles
STORAGE: Store at 36° - 77°F (2° - 25°C).
Rx Only
Alcon®
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
© 2002, 2013, 2015 Novartis
9012635-1115
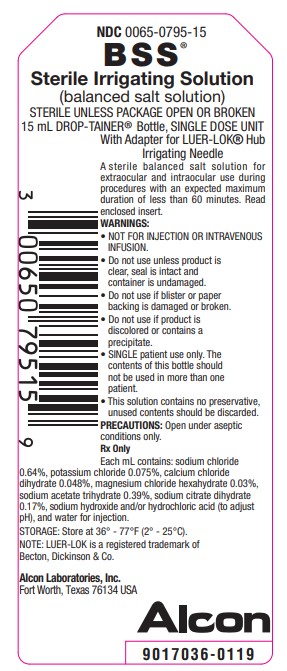
NDC 0065-0795-15
BSS®
Sterile Irrigating Solution
(balanced salt solution)
STERILE UNLESS PACKAGE OPEN OR BROKEN
15 mL DROP-TAINER® Bottle, SINGLE DOSE UNIT With Adapter for LUER-LOK® Hub Irrigating Needle
A sterile balanced salt solution for
extraocular and intraocular use during
procedures with an expected maximum
duration of less than 60 minutes. Read
enclosed insert.
WARNINGS:
• NOT FOR INJECTION OR INTRAVENOUSINFUSION.
• Do not use unless product is clear, seal is intact and container is undamaged.
• Do not use if blister or paper backing is damaged or broken.
• Do not use if product is discolored or contains a precipitate.
• SINGLE patient use only. The contents of this bottle should not be used in more than one patient.
• This solution contains no preservative, unused contents should be discarded.
PRECAUTIONS: Open under aseptic
conditions only.
Rx Only
Each mL contains: sodium chloride
0.64%, potassium chloride 0.075%, calcium chloride
dihydrate 0.048%, magnesium chloride hexahydrate 0.03%,
sodium acetate trihydrate 0.39%, sodium citrate dihydrate
0.17%, sodium hydroxide and/or hydrochloric acid (to adjust
pH), and water for injection.
STORAGE: Store at 36° - 77°F (2° - 25°C).
NOTE: LUER-LOK is a registered trademark of
Becton, Dickinson & Co.
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
Alcon
9017036-0119
NDC 0065-0795-15
BSS®
Sterile Irrigating Solution
(balanced salt solution)
STERILE UNLESS PACKAGE OPEN OR BROKEN
15 mL DROP-TAINER® Bottle, SINGLE DOSE UNIT With Adapter for LUER-LOK® Hub Irrigating Needle
A sterile balanced salt solution for extraocular and intraocular use during procedures with an expected maximum duration of less than 60 minutes. Read enclosed insert.
WARNINGS:
• NOT FOR INJECTION OR INTRAVENOUS INFUSION.
• Do not use unless product is clear, seal is intact and container is undamaged.
• Do not use if blister or paper backing is damaged or broken.
• Do not use if product is discolored or contains a precipitate.
• SINGLE patient use only. The contents of this bottle should not be used in more than one patient.
• This solution contains no preservative, unused contents should be discarded.
PRECAUTIONS: Open under aseptic conditions only.
Rx Only
Each mL contains: sodium chloride 0.64%, potassium chloride 0.075%, calcium chloride dihydrate 0.048%, magnesium chloride hexahydrate 0.03%, sodium acetate trihydrate 0.39%, sodium citrate dihydrate 0.17%, sodium hydroxide and/or hydrochloric acid (to adjust pH), and water for injection.
STORAGE: Store at 36° - 77°F (2° - 25°C).
NOTE: LUER-LOK is a registered trademark of Becton, Dickinson & Co.
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
©2003, 2013,2015 Novartis
Alcon®
a Novartis company
9012632-1115

NDC 0065-0795-15
BSS®
STERILE IRRIGATING SOLUTION
(BALANCED SALT SOLUTION)
UNIT DOSE 15 mL
Rx Only
ALCON LABORATRIES, INC.
FORT WORTH
TEXAS 76134 USA
289010-0902

-
INGREDIENTS AND APPEARANCE
BSS
balanced salt solution solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0065-0795 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 6.4 mg in 1 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORIDE 0.75 mg in 1 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CHLORIDE 0.48 mg in 1 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM CHLORIDE 0.3 mg in 1 mL SODIUM ACETATE (UNII: 4550K0SC9B) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM ACETATE 3.9 mg in 1 mL SODIUM CITRATE (UNII: 1Q73Q2JULR) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CITRATE 1.7 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0065-0795-15 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/28/1969 2 NDC:0065-0795-30 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/28/1969 12/31/2018 3 NDC:0065-0795-25 250 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/28/1969 02/28/2018 4 NDC:0065-0795-50 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/28/1969 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020742 03/28/1969 Labeler - Alcon Laboratories, Inc. (008018525) Registrant - Alcon Laboratories, Inc. (008018525) Establishment Name Address ID/FEI Business Operations Alcon Research LLC 007672236 manufacture(0065-0795) Establishment Name Address ID/FEI Business Operations S.A. Alcon-Couvreur N.V. 370205429 manufacture(0065-0795)





