Label: YF-VAX- yellow fever virus strain 17d-204 live antigen injection, powder, lyophilized, for suspension
DILUENT- sodium chloride injection
-
NDC Code(s):
49281-912-05,
49281-912-10,
49281-912-59,
49281-912-69, view more49281-915-01, 49281-915-05, 49281-915-58, 49281-915-68
- Packager: Sanofi Pasteur Inc.
- Category: VACCINE LABEL
Drug Label Information
Updated January 8, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
YF-VAX®, Yellow Fever Vaccine, for subcutaneous use, is prepared by culturing the 17D-204 strain of yellow fever virus in living avian leukosis virus-free (ALV-free) chicken embryos. The vaccine contains sorbitol and gelatin as a stabilizer, is lyophilized, and is hermetically sealed under nitrogen. No preservative is added. Each vial of vaccine is supplied with a separate vial of sterile diluent, which contains Sodium Chloride Injection USP – without a preservative. YF-VAX is formulated to contain not less than 4.74 log10 plaque forming units (PFU) per 0.5 mL dose throughout the life of the product. Before reconstitution, YF-VAX is a pinkish color. After reconstitution, YF-VAX is a slight pink-brown suspension.
The vial stoppers for YF-VAX and diluent are not made with natural rubber latex.
-
CLINICAL PHARMACOLOGY
Yellow fever is an acute viral illness caused by a mosquito-borne flavivirus. Most yellow fever virus infections are asymptomatic. In those individuals who develop disease, the clinical spectrum ranges from nonspecific flu-like illness with fever, malaise, prostration, headache, photophobia, generalized arthralgia and myalgia, nausea, and/or vomiting to potentially lethal pansystemic disease, most prominently involving the liver, kidneys, GI tract, and brain, with recrudescing fever, jaundice, renal failure, severe hemorrhage due to thrombocytopenia, and shock. (1) The case-fatality rate of yellow fever varies widely in different studies but is typically 20% or higher. Jaundice or other gross evidence of severe liver disease is associated with higher mortality rates.
Two live, attenuated yellow fever vaccines, strains 17D-204 and 17DD, were derived in parallel in the 1930s. Historical data suggest that these "17D vaccines" have identical safety and immunogenicity profiles. Vaccination with 17D strain vaccines is predicted to elicit an immune response identical in quality to that induced by wild-type infection. This response is presumed to result from initial infection of cells in the dermis or other subcutaneous tissues near the injection site, with subsequent replication and limited spread of virus leading to the processing and presentation of viral antigens to the immune system, as would occur during infection with wild-type yellow fever virus. The humoral immune response to the viral structural proteins, as opposed to a cell-mediated response, is most important in the protective effect induced by 17D vaccines. Yellow fever antibodies with specificities that prevent or abort infection of cells are detected as neutralizing antibodies in assays that measure the ability of serum to reduce plaque formation in tissue culture cells. The titer of virus neutralizing antibodies in sera of vaccinees is a surrogate for efficacy. A log10 neutralization index (LNI, measured by a plaque reduction assay) of 0.7 or greater was shown to protect 90% of monkeys from lethal intracerebral challenge. (2) This is the definition of seroconversion adopted for clinical trials of yellow fever vaccine. The standard has also been adopted by the World Health Organization (WHO) for efficacy of yellow fever vaccines in humans. (3)
In 24 uncontrolled studies conducted world-wide between 1962 and 1997 evaluating neutralizing antibody responses to 17D vaccines among a total of 2,529 adults and 991 infants and children, the seroconversion rate was greater than 91% in all but two studies and never lower than 81%. There were no significant age-related differences in immunogenicity. (1)
Five of these 24 studies were conducted in the US between 1962 and 1993 and included 208 adults who received YF-VAX. The seroconversion rate was 81% in one study involving 32 subjects and 97% to 100% in the other four studies. (1) (4) (5) (6) (7)
In 2001, YF-VAX was used as a control in a double-blind, randomized comparison trial with another 17D-204 vaccine, conducted at nine centers in the US. YF-VAX was administered to 725 adults ≥18 years old with a mean age of 38 years. Three hundred twelve of these subjects who received YF-VAX were evaluated serologically, and 99.3% of them seroconverted with a mean LNI of 2.21. The LNI was slightly higher among males compared to females and slightly lower among Hispanic and African-American subjects compared to others, but these differences were not associated with differences in protective effect of the vaccine. There was no difference in mean LNI for subjects <40 years old compared to subjects ≥40 years old. Due to the small number of subjects (1.7%) with prior flavivirus immunity, it was not possible to draw conclusions about the role of this factor in the immune response. (8)
For most healthy individuals, a single dose of yellow fever vaccine provides long-lasting protection. (9) (10) In controlled studies where the immune response to vaccination was evaluated, the small percentage of immunologically normal individuals who failed to develop an immune response to an initial vaccination typically did so upon re-vaccination. (11)
In two separate clinical trials of 17D-204 vaccines, 90% of subjects seroconverted within 10 days after vaccination, (12) and 100% of subjects seroconverted within 14 days. (1) Thus, International Health regulations stipulate that the vaccination certificate for yellow fever is valid 10 days after administration of YF-VAX. (13)
-
INDICATIONS AND USAGE
YF-VAX is indicated for active immunization for the prevention of yellow fever in persons 9 months of age and older in the following categories:
Persons Living in or Traveling to Endemic Areas
While the actual risk for contracting yellow fever during travel is probably low, variability of itineraries, behaviors and seasonal incidence of disease make it difficult to predict the actual risk for a given individual living in or traveling to a known endemic or epidemic area. Greater risk is associated with living in or traveling to areas of South America and Africa where yellow fever infection is officially reported at the time of travel and with traveling outside the urban areas of countries that do not officially report the disease but that lie in a yellow fever endemic zone.
Persons Traveling Internationally Through Countries with Yellow Fever
Some countries require an individual to have a valid International Certificate of Vaccination or Prophylaxis (ICVP) if the individual has been in countries either known or thought to harbor yellow fever virus. The certificate becomes valid 10 days after vaccination with YF-VAX. (13) (14)
-
CONTRAINDICATIONS
Hypersensitivity
YF-VAX is contraindicated in anyone with a history of acute hypersensitivity reaction to any component of the vaccine. (See DESCRIPTION section.) Because the yellow fever virus used in the production of this vaccine is propagated in chicken embryos, do not administer YF-VAX to anyone with a history of acute hypersensitivity to eggs or egg products due to a risk of anaphylaxis. Less severe or localized manifestations of allergy to eggs or to feathers are not contraindications to vaccine administration and do not usually warrant vaccine skin testing. (See PRECAUTIONS section, Testing for Hypersensitivity Reactions subsection.) Generally, persons who are able to eat eggs or egg products may receive the vaccine. (14) (15)
Individuals Less Than 9 Months of Age
Vaccination with YF-VAX is contraindicated in infants less than 9 months of age due to an increased risk of encephalitis.
Vaccination with YF-VAX is also contraindicated in lactating women who are providing breastmilk to infants less than 9 months of age due to the potential for transmission of vaccine virus in breastmilk. (See PRECAUTIONS section, Nursing Mothers subsection.)
Immunosuppressed Individuals
Vaccination with YF-VAX, a live virus vaccine, is contraindicated in individuals with severe immunosuppression, including for example, those with acquired immunodeficiency syndrome, leukemia, lymphoma, thymic disease, generalized malignancy, and patients who are undergoing drug therapy (e.g., systemic corticosteroids, alkylating drugs, antimetabolites or other immunomodulatory drugs) or radiation therapy. Thymic disorders associated with abnormal immune cell function (e.g., myasthenia gravis, thymoma) may be an independent risk factor for the development of yellow fever vaccine-associated viscerotropic disease. (See WARNINGS section.) (16)
Do not administer YF-VAX to individuals with severe immunosuppression.
Family members of immunosuppressed persons, who themselves have no contraindications, may receive YF-VAX. (14) (17)
-
WARNINGS
Severe Allergic Reactions
Severe allergic reactions (e.g., anaphylaxis) may occur following the use of YF-VAX, even in individuals with no prior history of hypersensitivity to the vaccine components. Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of the vaccine.
Yellow fever vaccine-associated viscerotropic disease
Age greater than 60 years is a risk factor for yellow fever vaccine-associated viscerotropic disease (YEL-AVD) (14) which may present as non-specific multi-organ system failure or can be similar to fulminant yellow fever caused by wild-type yellow fever virus, with liver failure and internal bleeding, leading to death. (See ADVERSE REACTIONS section.) Available evidence suggests that the occurrence of this syndrome may depend upon undefined host factors, rather than intrinsic virulence of the yellow fever strain 17D vaccine, based on characterization of vaccine viruses isolated from individuals with YEL-AVD. YEL-AVD has been reported to occur only after the first dose of yellow fever vaccine; there have been no reports of YEL-AVD following booster dose. (17) The decision to vaccinate individuals 60 years of age and older needs to weigh the risks and benefits of vaccination and the risk for exposure to yellow fever virus. (18) (19) (20) (21)
Yellow fever vaccine-associated neurotropic disease
Age greater than 60 years and immunosuppression are risk factors for post-vaccinal encephalitis, also known as yellow fever vaccine-associated neurotropic disease (YEL-AND). (See ADVERSE REACTIONS section.) Almost all cases of YEL-AND have been in first-time vaccine recipients. (17) The decision to vaccinate individuals 60 years of age and older and immunosuppressed individuals needs to weigh the risks and benefits of vaccination and the risk for exposure to yellow fever virus.
-
PRECAUTIONS
General
Vaccination with YF-VAX may not protect 100% of individuals.
Do not administer YF-VAX by intravascular, intramuscular, or intradermal routes.
Use a separate, sterile syringe and needle for each patient to prevent transmission of blood borne infectious agents. Do not recap needles. Dispose of needles and syringes according to biohazard waste guidelines.
Testing for Hypersensitivity Reactions
Do not administer YF-VAX to an individual with a history of hypersensitivity to egg or chicken protein. (See CONTRAINDICATIONS section.) However, if an individual is suspected of being an egg-sensitive individual, the following test can be performed before the vaccine is administered:
1. Scratch, prick, or puncture test
Place a drop of a 1:10 dilution of the vaccine in physiologic saline on a superficial scratch, prick, or puncture on the volar surface of the forearm. Positive (histamine) and negative (physiologic saline) controls should also be used. The test is read after 15 to 20 minutes. A positive test is a wheal (superficial bump) 3 mm larger than that of the saline control, usually with surrounding erythema. The histamine control must be positive for valid interpretation. If the result of this test is negative, an intradermal (ID) test should be performed.
2. Intradermal test
Inject a dose of 0.02 mL of a 1:100 dilution of the vaccine in physiologic saline. Positive and negative control skin tests should be performed concurrently. A wheal 5 mm or larger than the negative control with surrounding erythema is considered a positive reaction.
If vaccination is considered essential despite a positive skin test, consider desensitization. (See DOSAGE AND ADMINISTRATION section, Desensitization subsection.)
Syncope
Syncope can occur following or even before vaccination. Procedures should be in place to prevent falling and injury and to manage syncope.
Information for Patients
Prior to administration of YF-VAX, ask potential vaccinees or their parents or guardians about their recent health status and history of yellow fever vaccination. Inform potential vaccinees or their parents or guardians about the benefits and risks of immunization and potential for adverse reactions to YF-VAX administration. Instruct vaccinees or their parents or guardians to report to their health-care providers all serious adverse events that occur up to 30 days post-vaccination.
All travelers should seek information regarding vaccination requirements by consulting with their health care providers. Such requirements may be strictly enforced for entry into certain countries, particularly for persons traveling from Africa or South America to Asia. Additional information is available from local health departments, the Centers for Disease Control and Prevention (CDC), and WHO. Travel agencies, international airlines, and/or shipping lines may also have up-to-date information. The vaccination center should complete, sign, and stamp an International Certificate of Vaccination and provide the certificate to the vaccinee. The immunization record should contain the date, lot number and manufacturer of the vaccine administered. Inform vaccinees that vaccination certificates are valid commencing 10 days after vaccination. (14)
Drug Interactions
Data are limited in regard to the interaction of YF-VAX with other vaccines.
- Measles (Schwartz strain) vaccine, diphtheria and tetanus toxoids and whole cell pertussis vaccine (DTP), (22) Hepatitis A and Hepatitis B vaccines, (5) (14) (23) (24) meningococcal vaccine, Menomune®A/C/Y/W-135, and typhoid vaccine, Typhim Vi®, (5) (14) (23) have been administered with yellow fever vaccine at separate injection sites.
- The potential for interference between yellow fever vaccine and rabies or Japanese encephalitis vaccines has not been established. (14)
- In a prospective study, persons given 5 cc of commercially available immune globulin did not experience alterations in immunologic responses to the yellow fever vaccine. (14) (25) (26)
- Although chloroquine inhibits replication of yellow fever vaccine in vitro, it does not appear to adversely affect antibody responses to yellow fever vaccine among persons receiving chloroquine. (14) (27)
Patients on Corticosteroid Therapy
Oral Prednisone or other systemic corticosteroid therapy, depending on dose and duration of exposure, may have an immunosuppressive effect on recipients of yellow fever vaccine that potentially decreases immunogenicity and increases the risk of adverse events. Intra-articular, bursal, or tendon injections with corticosteroids should not constitute an increased hazard to recipients of yellow fever vaccine.
Patients with Asymptomatic Human Immunodeficiency Virus (HIV) Infection
The rate of seroconversion following YF-VAX is reduced in individuals with asymptomatic HIV infection and appears to depend on HIV viral load and CD4 + T-cell count. (14) Therefore, documentation of a protective antibody response is recommended before travel. (See CLINICAL PHARMACOLOGY section.) For discussion of this subject and for documentation of the immune response to vaccine where it is deemed essential, contact the CDC at 1-970-221-6400.
Carcinogenesis, Mutagenesis, Impairment of Fertility
YF-VAX has not been evaluated for its carcinogenic or mutagenic potential or its effect on fertility.
Pregnancy
Animal reproduction studies have not been conducted with YF-VAX. It is also not known whether YF-VAX can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. YF-VAX should be given to a pregnant woman only if clearly needed.
YF-VAX has not been evaluated in pregnant women. However, based on experience of other yellow fever vaccines, the following findings have been determined for safety and effectiveness. A case-control study of Brazilian women found no significant difference in the odds ratio of spontaneous abortion among vaccinated women compared to a similar unvaccinated group. (28) In a separate study in Trinidad, 100 to 200 pregnant females were immunized, no adverse events related to pregnancy were reported. In addition, 41 cord blood samples were obtained from infants born to mothers immunized during the first trimester. One of these infants tested positive for IgM antibodies in cord blood. The infant appeared normal at delivery, and no subsequent adverse sequelae of infection were reported. However, this result suggests that transplacental infection with 17D vaccine viruses can occur. (29) In another study involving 101 Nigerian women, the majority of whom (88%) were in the third trimester of pregnancy, none of the 40 infants who were delivered in a hospital tested positive for IgM antibodies as a criterion for transplacental infection with vaccine virus. However, the percentage of pregnant women who seroconverted was reduced compared to a non-pregnant control group (38.6% vs. 81.5%). (30)
For further discussion of vaccination with YF-VAX during pregnancy and for documentation of a protective immune response to vaccine where it is deemed essential, contact the CDC at 1-970-221-6400.
Nursing Mothers
Because of the potential for serious adverse reactions in nursing infants from YF-VAX, a decision should be made whether to discontinue nursing or not to administer the vaccine, taking into account the importance of the vaccine to the mother. As of July, 2015, three vaccine-associated neurotropic disease cases have been reported worldwide in exclusively breastfed infants whose mothers were vaccinated with yellow fever vaccines, including one case reported after vaccination with YF-VAX. All three infants were diagnosed with encephalitis and were less than one month of age at the time of exposure. (17) Because age less than 9 months is a risk factor for yellow fever vaccine-associated neurotropic disease, YF-VAX is contraindicated in lactating women who are providing breastmilk to infants younger than 9 months of age. (See CONTRAINDICATIONS section.) Discuss the risks and benefits of vaccination with lactating women who are providing breastmilk to infants 9 months of age and older. (14)
Pediatric Use
Vaccination of infants less than 9 months of age is contraindicated because of the risk of yellow fever vaccine-associated neurotropic disease. (See CONTRAINDICATIONS and ADVERSE REACTIONS sections.)
Geriatric Use
There is an increased risk of severe systemic adverse reactions to YF-VAX in individuals 60 years of age and older. Monitor elderly individuals for signs and symptoms of yellow fever vaccine-associated viscerotropic disease, which typically occurs within 10 days post-vaccination. (See WARNINGS and ADVERSE REACTIONS sections.) (16) (31)
-
ADVERSE REACTIONS
Data from Clinical Studies
Adverse reactions to YF-VAX include mild headaches, myalgia, low-grade fevers, or other minor symptoms for 5 to 10 days. Local reactions including edema, hypersensitivity, pain or mass at the injection site have also been reported following yellow fever vaccine administration. Immediate hypersensitivity reactions, characterized by rash, urticaria, and/or asthma, occur principally among persons with histories of allergy to eggs or other substances contained in the vaccine.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
No placebo-controlled trial has assessed the safety of YF-VAX. However, between 1953 and 1994, reactogenicity of 17D-204 vaccine was monitored in 10 uncontrolled clinical trials. The trials included a total of 3,933 adults and 264 infants greater than 4 months old residing in Europe or in yellow fever endemic areas. Self-limited and mild local reactions consisting of erythema and pain at the injection site and systemic reactions consisting of headache and/or fever occurred in a minority of subjects (typically less than 5%) 5 to 7 days after immunization. In one study involving 115 infants age 4 to 24 months the incidence of fever was as high as 21%. Also in this study, reactogenicity of the vaccine was markedly reduced among a subset of subjects who had serological evidence of previous exposure to yellow fever virus. Only two of the ten studies provided diary cards for daily reporting; this method resulted in a slightly higher incidence of local and systemic complaints. YF-VAX was used as a control in a double-blind, randomized comparative trial with another 17D-204 vaccine, conducted at nine centers in the US. YF-VAX was administered to 725 adults ≥18 years old with a mean age of 38 years. Safety data were collected by diary card for days 1 through 10 after vaccination and by interview on days 5, 11, and 31. Among subjects who received YF-VAX, there were no serious adverse events, and 71.9% experienced non-serious adverse events judged to have been related to vaccination. Most of these were injection site reactions of mild to moderate severity. Four such local reactions were considered severe. Rash occurred in 3.2%, including two subjects with urticaria. Systemic reactions (headache, myalgia, malaise, and asthenia) were usually mild and occurred in 10% to 30% of subjects during the first few days after vaccination. The incidence of non-serious adverse reactions, including headache, malaise, injection site edema, and pain, was significantly lower in subjects >60 years compared to younger subjects. Adverse events were less frequent in the 1.7% of vaccinated subjects who had pre-existing immunity to yellow fever virus, compared to those without pre-existing immunity. (8)
Data from Post-marketing Experience
The following additional adverse events have been spontaneously reported during the post-marketing use of YF-VAX worldwide. Because these events are reported voluntarily from a population of uncertain size, it is not possible to estimate their frequency reliably or establish a causal relationship to vaccine exposure. This list includes adverse events based on one or more of the following factors: severity, frequency of reporting, or strength of evidence for a causal relationship to YF-VAX.
• Immune System Disorders (14)
Immediate hypersensitivity reactions or anaphylaxis, characterized by rash and/or urticaria and/or respiratory symptoms (e.g., dyspnea, bronchospasm, or pharyngeal edema) occur principally among persons with histories of allergies to egg or other substances contained in the vaccine.
• Nervous System Disorders (1) (32) (33) (34)
Isolated cases of Yellow Fever Vaccine-Associated Neurotropic Disease (YEL-AND), sometimes fatal, have been reported to occur within 30 days following vaccination with YF-VAX, and other yellow fever vaccines. (See WARNINGS section, Yellow fever vaccine-associated neurotropic disease subsection.) Age less than 9 months and congenital or acquired immunodeficiency have been identified as risk factors for this event. (See WARNINGS and CONTRAINDICATIONS sections.) Twenty-one cases of YEL-AND associated with all licensed 17D vaccines have been reported between 1952 and 2004. Eighteen of these cases were in children or adolescents. Fifteen of these cases occurred prior to 1960, thirteen of which occurred in infants 4 months of age or younger, and two of which occurred in infants six and seven months old. The incidence of vaccine-associated neurologic disease in infants less than 4 months old is estimated to be between 50 and 400 cases per 1,000,000, based on two historical reports where denominators are available. (33) (34) (35) A study in Senegal (34) described two fatal cases of encephalitis possibly associated with 17D-204 vaccination among 67,325 children between the ages of 6 months and 2 years, for an incidence rate of 3 per 100,000. The incidence of YEL-AND in the United States is less than 1:100,000 doses administered. (17)
Other neurological complications have included Guillain-Barré syndrome (GBS), acute disseminated encephalomyelitis (ADEM), and bulbar palsy.
• Infections and infestations
Isolated cases of Yellow Fever Vaccine-Associated Viscerotropic Disease YEL-AVD, formerly described as "Febrile Multiple Organ-System-Failure", sometimes fatal, have been reported following YF-VAX and other yellow fever vaccines. (See WARNINGS section, Yellow fever vaccine-associated viscerotropic disease subsection.) In the majority of cases reported, the onset of signs and symptoms was within 10 days after the vaccination. Initial signs and symptoms are non-specific and may include pyrexia, myalgia, fatigue and headache, potentially progressing quickly to liver and muscle cytolysis and possibly to thrombocytopenia, lymphopenia and acute renal failure. (18) The pathophysiological mechanism of such reactions has not been established. In some individuals with YEL-AVD a medical history of thymic disease has been reported. (36) Age older than 60 has also been identified as a risk factor for this event. (9) During surveillance in the U.S. between 1996 and 1998, four individuals (ages 63, 67, 76, and 79) became severely ill 2 to 5 days after vaccination with YF-VAX vaccine. Three of these 4 subjects died. The incidence rate for these serious adverse events was estimated at 1 per 400,000 doses of YF-VAX vaccine, based on the total number of doses administered in the U.S. civilian population during the surveillance period. (21) YEL-AVD has occurred after yellow fever vaccination in fewer than 1:100,000 U.S. vaccinees, (14) most commonly in individuals 60 years of age and older.
In a CDC analysis of data submitted to the Vaccine Adverse Events Reporting System (VAERS) between 1990 and 1998, the rate of systemic adverse events following vaccination was 2.5-fold higher in the 65 years or older age group (6.2 events per 100,000 doses of vaccine) compared to the 25 to 44 year-old age group (2.5 events per 100,000 doses of vaccine). (31)
-
DOSAGE AND ADMINISTRATION
Primary Vaccination
Administer a single subcutaneous injection of 0.5 mL of reconstituted vaccine.
Additional Dosing Information
A single dose of yellow fever vaccine provides long-lasting protection to most healthy individuals. (See CLINICAL PHARMACOLOGY section.) However, an additional dose of yellow fever vaccine may be given to individuals who might not have had an adequate or sustained immune response to prior yellow fever vaccination and who continue to be at risk for exposure to yellow fever virus. Such individuals include women who were vaccinated during pregnancy, hematopoietic stem cell transplant recipients, and HIV-infected persons.
Booster Vaccination
A booster dose may be given to individuals who were last vaccinated against yellow fever at least 10 years prior and who are at increased risk for yellow fever disease either because of location and duration of travel or because of more consistent exposure to virulent virus. Such individuals include travelers who plan to spend a prolonged period in endemic areas or who plan to travel to highly endemic areas such as rural West Africa, and laboratory personnel who handle virulent yellow fever virus or concentrated preparations of the yellow fever vaccine virus strains. (10)
Some countries may require for entry evidence of a valid yellow fever vaccination (i.e., ICVP) within the previous 10 years for certain individuals, depending on prior travel itinerary. A booster dose of YF-VAX may be given to satisfy this requirement. (10) (37)
Concomitant Administration with other Vaccines
Limited data are available related to administration of YF-VAX with other vaccines and the potential for immune interference. (See PRECAUTIONS section, Drug Interactions subsection.) In instances where vaccines are given concomitantly, administer injections using separate syringes at separate sites. Do not combine or mix YF-VAX with any other vaccine. When not administered concomitantly, wait at least 4 weeks between administration of YF-VAX and other live vaccines. (14)
Vaccine Preparation
- Reconstitute the vaccine using only the diluent supplied (0.6 mL single dose vial of Sodium Chloride Injection USP for single dose vial of vaccine). After removing the "flip-off" caps, cleanse the vaccine and diluent vial stoppers with a suitable germicide. Do not remove the vial stoppers or metal seals holding them in place. Using aseptic technique, use a suitable sterile needle and syringe to withdraw the volume of supplied diluent shown on the diluent label and slowly inject the diluent into the vial containing the vaccine. Allow the reconstituted vaccine to sit for one to two minutes and then carefully swirl mixture until a uniform suspension is achieved. Avoid vigorous shaking as this tends to cause foaming of the suspension. Do not dilute reconstituted vaccine. Use aseptic technique and a separate sterile needle and syringe to withdraw each 0.5 mL dose from the single dose vial of reconstituted vaccine.
- Before reconstitution, YF-VAX is a pinkish color. After reconstitution, YF-VAX is a slight pink-brown suspension. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exists, do not administer the vaccine.
- Administer the single dose of 0.5 mL subcutaneously using a suitable sterile needle.
- Use YF-VAX within 60 minutes of reconstituting the single dose vial.
- Discard unused portion.
Properly dispose of all reconstituted vaccine and containers that remain unused after one hour according to locally approved guidelines (e.g. sterilized or disposed in red hazardous waste containers). (14)
Desensitization
If immunization is imperative and the individual has a history of severe egg sensitivity and has a positive skin test to the vaccine, this desensitization procedure may be used to administer the vaccine. The following successive doses should be administered subcutaneously at 15 to 20 minute intervals:
- 0.05 mL of 1:10 dilution
- 0.05 mL of full strength
- 0.10 mL of full strength
- 0.15 mL of full strength
- 0.20 mL of full strength
Desensitization should only be performed under the direct supervision of a physician experienced in the management of anaphylaxis with necessary emergency equipment immediately available.
-
HOW SUPPLIED
The vial stoppers for YF-VAX vaccine and diluent are not made with natural rubber latex.
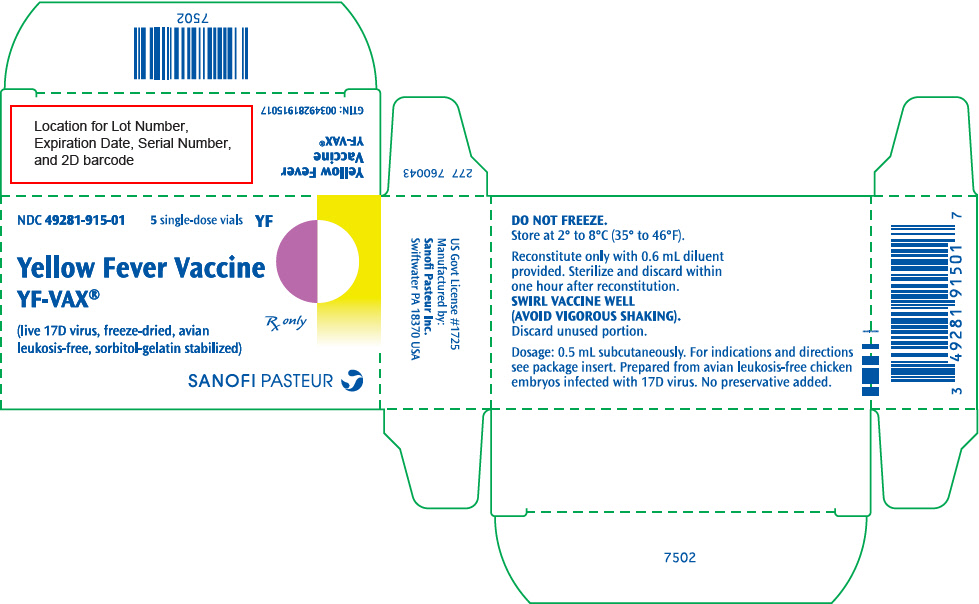
Vaccine, single dose vial (NDC 49281-915-58) supplied in a package of 5 vials (NDC 49281-915-01).
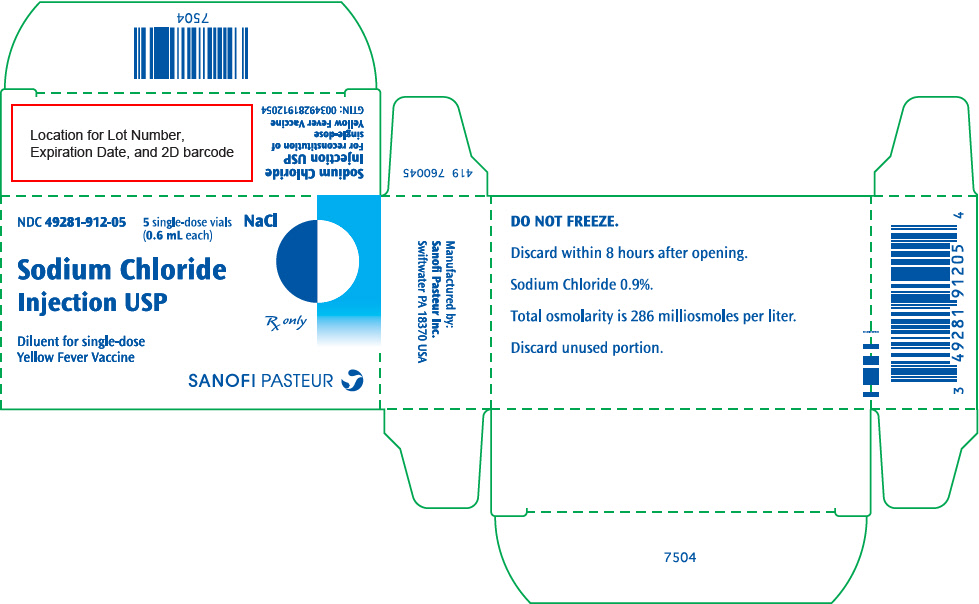
Diluent, single dose vial, 0.6 mL (NDC 49281-912-59) supplied separately in a package of 5 vials (NDC 49281-912-05).
YF-VAX (Yellow Fever Vaccine) in the US is supplied only to designated Yellow Fever Vaccination Centers authorized to issue certificates of Yellow Fever Vaccination. Location of the nearest Yellow Fever Vaccination Centers may be obtained from the Centers for Disease Control and Prevention, Atlanta, GA 30333, state or local health departments. STORAGE
Store at 2° to 8°C (35° to 46°F). DO NOT FREEZE.
Do not use vaccine after expiration date. YF-VAX does not contain a preservative.
The following stability information for YF-VAX is provided for those countries or areas of the world where an adequate cold chain is a problem and inadvertent exposure to abnormal temperatures has occurred. Half-life is reduced from approximately 14 days at 35° to 37°C to 3-4.5 days at 45° to 47°C.
-
REFERENCES
- 1
- Monath TP et al. Yellow fever vaccine. In: Plotkin SA, Orenstein WA and Offit PA, eds. Vaccines. 6th Ed. Elsevier Saunders Inc. 2013:870-968.
- 2
- Mason RA, et al. Yellow fever vaccine: Direct challenge of monkeys given graded doses of 17D vaccine. Appl Microbiol 1973;25(4):539-44.
- 3
- Recommendations to assure the quality, safety and efficacy of live attenuated yellow fever vaccines. WHO Technical Report Series. 2013;978:264.
- 4
- Wisseman CL, et al. Immunological studies with Group B arthropod-borne viruses. Am J Trop Med Hyg 1962;11:550-61.
- 5
- Dukes C, et al. Safety and Immunogenicity of Simultaneous Administration of Typhim Vi (TV), YF-VAX (YV), and Menomune (MV). [abstract]. American Society for Microbiology. The 36th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC): 1996; September 15-18:159.
- 6
- Meyer HM, et al. Response of Volta children to jet inoculation of combined live measles, smallpox, and yellow fever vaccines. Bull World Health Org 1964;30:783-94.
- 7
- Jackson J, et al. Comparison of Antibody Response and Patient Tolerance of Yellow Fever Vaccine Administered by the Bioject Needle-Free Injection System versus Conventional Needle/Syringe Injection. Third International Conference on Travel Medicine; Paris 1993;April:25-29;264:209.
- 8
- Monath TP, et al. Comparative safety and immunogenicity of two yellow fever 17D vaccines (ARILVAX and YF-VAX) in a Phase III multicenter, double-blind clinical trial. Am J Trop Med Hyg 66(5)2002;533-41.
- 9
- World Health Organization (WHO). Yellow fever vaccine - position paper. Wkly Epid Rec 2003;40(78):349-60.
- 10
- Staples JE et al. Yellow Fever Vaccine Booster Doses: Recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR 2015;64(23):647-50.
- 11
- Bonnevie-Nielson V, et al. Lymphocytic 2',5' - Oligoadenylate synthetase activity increases prior to the appearance of neutralizing antibodies and Immunoglobulin M and Immunoglobulin G antibodies after primary and secondary immunization with yellow fever vaccine. Clin Diag Lab Immunol 1995;2:302-6.
- 12
- Smithburn KC, et al. Immunization against yellow fever: Studies on the time of development and the duration of induced immunity. Am J Trop Med Page 7 of 8 Hyg 1945;45:217-23.
- 13
- World Health Organization (WHO). International Health Regulations (2005) (2nd edition). Geneva 2008:54-5.
- 14
- Recommendations of the Advisory Committee on Immunization Practices (ACIP). Yellow Fever Vaccine. MMWR 2010;59(RR-7):1-32.
- 15
- Centers for Disease Control and Prevention. General Recommendations on Immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011;60(No. RR2):(1-64).
- 16
- Sanofi Pasteur Inc. Data on File – 080601;120104.
- 17
- Centers for Disease Control and Prevention. CDC Health Information for International Travel 2016. New York: Oxford University Press 2016;3:346-60.
- 18
- Martin M, et al. Fever and multisystem organ failure associated with 17D-204 yellow fever vaccination: a report of four cases. Lancet 2001;358:98-104.
- 19
- Galler R, et al. Phenotypic and molecular analyses of yellow fever 17DD vaccine viruses associated with serious adverse events in Brazil. Virology 2001;290:309-19.
- 20
- Chan RC, et al. Hepatitis and death following vaccination with yellow fever 17D-204 vaccine. Lancet 2001;358:121-2.
- 21
- Vasconcelos PFC, et al. Serious adverse events associated with yellow fever 17DD vaccine in Brazil: a report of two cases. Lancet 2001;358:91-7.
- 22
- Ruben FL, et al. Simultaneous administration of smallpox, measles, yellow fever, and diphtheria-pertussis-tetanus antigens to Nigerian children. Bull WHO 1973;48:175-81.
- 23
- Dumas R, et al. Safety and immunogenicity of a new inactivated hepatitis A vaccine and concurrent administration with a typhoid fever vaccine or a typhoid fever + yellow fever vaccine. Adv Therapy 1997;14:160-7.
- 24
- Coursaget P, et al. Simultaneous injection of plasma-derived or recombinant hepatitis B vaccines with yellow fever and killed polio vaccines. Vaccine 1995;13:109-11.
- 25
- Kaplan JE, et al. The effect of immune globulin on the response to trivalent oral poliovirus and yellow fever vaccinations. Bull WHO 1984;62(4):585-90.
- 26
- Edupuganti S, et al. A Randomized, Double-Blind, Controlled Trial of the 17D Yellow Fever Virus Vaccine Given in Combination with Immune Globulin or Placebo: Comparative Viremia and Immunogenicity. Am J Trop Med Hyg 2013;88(1):172-7.
- 27
- Tsai TF, et al. Chloroquine does not adversely affect the antibody response to yellow fever vaccine. J Infect Dis 1986;154(4):726-7.
- 28
- Nishioka SA, et al. Yellow fever vaccination during pregnancy and spontaneous abortion: a case-control study. Trop Med Int Health 1998;3(1):29-33.
- 29
- Tsai TF, et al. Congenital yellow fever virus infection after immunization in pregnancy. J Infect Dis 1993;168:1520-1523.
- 30
- Nasidi A, et al. Yellow fever vaccination and pregnancy: a four-year prospective study. Transactions of the Royal Society of Tropical Medicine and Hygiene 1993;87:337-9.
- 31
- Martin M, et al. Advanced age a risk factor for illness temporally associated with yellow fever vaccination. Emerg Infect Dis 2001;7:945-51.
- 32
- Jennings AD, et al. Analysis of a yellow fever virus isolated from a fatal case of vaccine-associated human encephalitis. J Infect Dis 1994;169:512-8.
- 33
- Louis JJ, et al. A case of encephalitis after 17D strain yellow fever vaccination. Pediatr 1981;36(7):547-50.
- 34
- Rey M, et al. Epidemiological and clinical aspects of encephalitis following yellow fever vaccination. Bull Soc Méd Afr Noire Lgue fr 1966;v XI,(3),560-74.
- 35
- Stuart G. Reactions following vaccination against yellow fever. In Smithburn KC, Durieux C, Koerber R, et al (eds.). Yellow Fever Vaccination. Geneva, WHO 1956;143-189.
- 36
- Data on file at sanofi pasteur. Global Pharmacovigilance Department Rationale for adding 'thymic disease' in the CCDS of AvP France Yellow Fever Vaccine Nov 2004.
- 37
- World Health Organization (WHO). Vaccines and vaccination against yellow fever. WHO position paper - June 2013. Wkly Epid Rec 2013;27(88):269-84.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 0.5 mL Vial Label
- PRINCIPAL DISPLAY PANEL - 5 Vial Package
- PRINCIPAL DISPLAY PANEL - 0.6 mL Vial Label
- PRINCIPAL DISPLAY PANEL - 5 Vial Package
-
INGREDIENTS AND APPEARANCE
YF-VAX
yellow fever virus strain 17d-204 live antigen injection, powder, lyophilized, for suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:49281-915 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength YELLOW FEVER VIRUS STRAIN 17D-204 LIVE ANTIGEN (UNII: PY4EET359T) (YELLOW FEVER VIRUS STRAIN 17D-204 LIVE ANTIGEN - UNII:PY4EET359T) YELLOW FEVER VIRUS STRAIN 17D-204 LIVE ANTIGEN 4.74 [PFU] in 0.5 mL Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49281-915-01 5 in 1 PACKAGE 1 NDC:49281-915-58 0.5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 2 NDC:49281-915-05 1 in 1 PACKAGE 2 NDC:49281-915-68 2.5 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103915 05/22/1953 DILUENT
sodium chloride injectionProduct Information Product Type VACCINE Item Code (Source) NDC:49281-912 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 4.5 mg in 0.5 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49281-912-05 5 in 1 PACKAGE 1 NDC:49281-912-59 0.6 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 2 NDC:49281-912-10 1 in 1 PACKAGE 2 NDC:49281-912-69 3 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103915 05/22/1953 Labeler - Sanofi Pasteur Inc. (086723285) Establishment Name Address ID/FEI Business Operations Sanofi Pasteur Inc. 086723285 MANUFACTURE