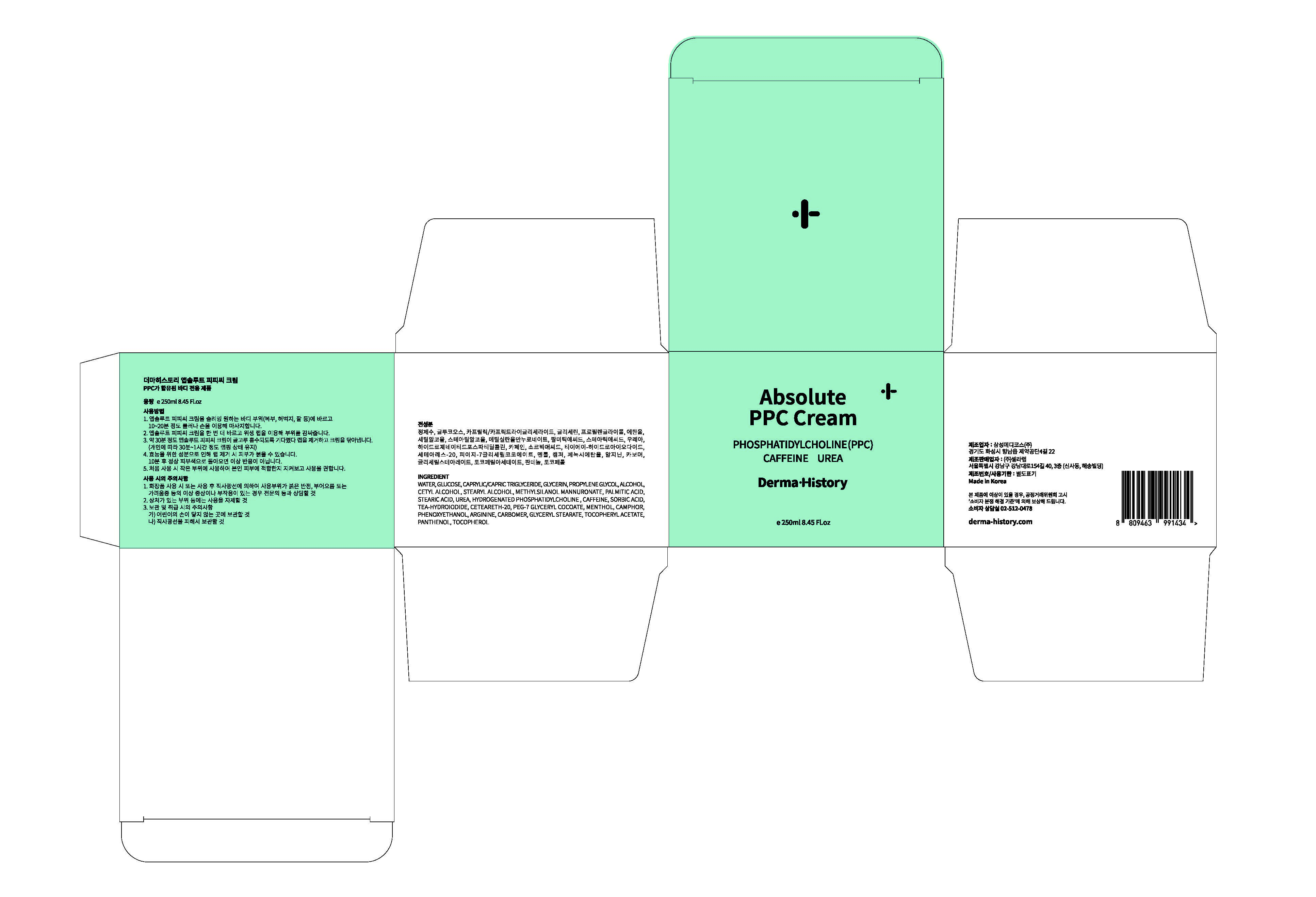
Label: ABSOLUTE PPC- glycerin cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 72312-0020-1 - Packager: CELLALAB
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 6, 2018
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
1. Apply PPC magic Cream on the body parts (abdomen, thigh, arms) where you want slimming. Massage the area either with your hand or roller for 10~20 minutes.
2. Apply PPC magic cream one more time and wrap the area with a plastic wrap.
3. Wait for 30 minutes for better absorption. Then, remove the wrap and wipe the cream off.(maintain the wrapping for 30min.~1hour depending on individual condition)
4. Due to the ingredients for efficacy, the skin may be red when removing the wrap. If it returns to normal skin color after 10 minutes, it is not an adverse reaction.
5. It is recommended to use in small areas for the first time to see if it is suitable for your skin. - WARNINGS
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ABSOLUTE PPC
glycerin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72312-0020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 2 g in 100 mL Inactive Ingredients Ingredient Name Strength UREA (UNII: 8W8T17847W) CAFFEINE (UNII: 3G6A5W338E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72312-0020-1 250 mL in 1 JAR; Type 0: Not a Combination Product 10/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part347 10/01/2018 Labeler - CELLALAB (690452262) Registrant - CELLALAB (690452262) Establishment Name Address ID/FEI Business Operations SAMSUNG MEDICOS. CO., LTD. Hyangnam Factory 689851701 manufacture(72312-0020) Establishment Name Address ID/FEI Business Operations CELLALAB 690452262 relabel(72312-0020)