Label: OLOPATADINE HYDROCHLORIDE solution
- NDC Code(s): 58602-012-40
- Packager: Aurohealth LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated August 21, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if you experience:
- Keep Out of Reach of Children.
-
Directions
-
adults and children 2 years of age and older:
- put 1 drop in the affected eye(s) twice daily, every 6 to 8 hours, no more than twice per day
- if using other ophthalmic products while using this product, wait at least 5 minutes between each product
- replace cap after each use
- children under 2 years of age:
consult a doctor
-
adults and children 2 years of age and older:
- Other information
- Inactive ingredients
- Questions?
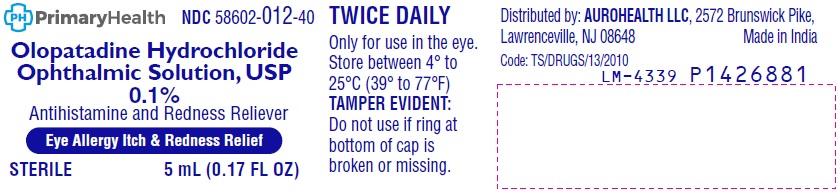
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL-0.1% (5 mL Container)
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL-0.1% (5 mL Container Carton)
NDC 58602-012-40
PrimaryHealth
*Compare to the Active Ingredient
in Pataday® Once Daily Relief
NOW AVAILABLE without a prescription
Olopatadine Hydrochloride
Ophthalmic Solution, USP
0.1%
Antihistamine and Redness Reliever
Eye Allergy Itch & Redness Relief
Works in Minutes
Relief from Allergens:
• Pet Dander • Pollen TWICE
• Grass • Ragweed DAILY
STERILE
5 mL (0.17 FL OZ)

-
INGREDIENTS AND APPEARANCE
OLOPATADINE HYDROCHLORIDE
olopatadine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58602-012 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OLOPATADINE HYDROCHLORIDE (UNII: 2XG66W44KF) (OLOPATADINE - UNII:D27V6190PM) OLOPATADINE HYDROCHLORIDE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58602-012-40 1 in 1 CARTON 07/15/2020 1 5 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204812 07/15/2020 Labeler - Aurohealth LLC (078728447) Registrant - Aurobindo Pharma Limited (650082092) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 650498244 ANALYSIS(58602-012) , MANUFACTURE(58602-012)