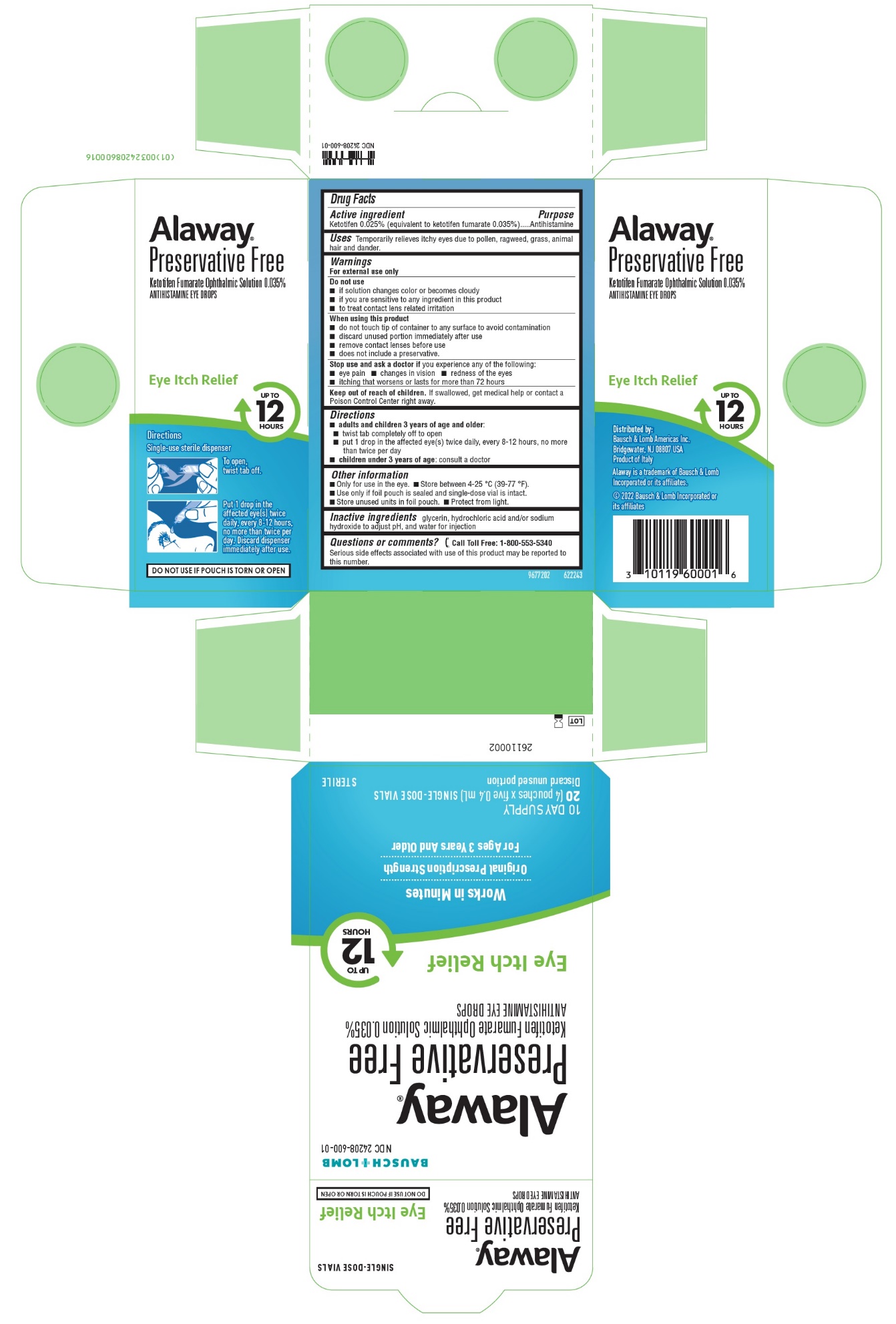
Label: ALAWAY PRESERVATIVE FREE- ketotifen fumarate solution/ drops
- NDC Code(s): 24208-600-01, 24208-600-99
- Packager: Bausch & Lomb Incorporated
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated May 4, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- •
- if solution changes color or becomes cloudy
- •
- if you are sensitive to any ingredient in this product
- •
- to treat contact lens related irritation
When using this product
- •
- do not touch tip of container to any surface to avoid contamination
- •
- discard unused portion immediately after use
- •
- remove contact lenses before use
- •
- does not include a preservative.
Stop use and ask a doctor if you experience any of the following:
- •
- eye pain
- •
- changes in vision
- •
- redness of the eyes
- •
- itching that worsens or lasts for more than 72 hours
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
-
Questions or comments?
[phone icon]Call Toll Free: 1-800-553-5340
Serious side effects associated with use of this product may be reported to this number.Distributed by:
Bausch & Lomb Americas Inc.
Bridgewater, NJ 08807 USA
Product of Italy
Alaway is a trademark of Bausch & Lomb
Incorporated or its affiliates.
© 2022 Bausch & Lomb Incorporated or its affiliates
9677202
622243
-
Package/Label Principal Display Panel
BAUSCH + LOMB
NDC 24208-600-01
Alaway®
Preservative FreeKetotifen Fumarate Ophthalmic Solution 0.035%
ANTIHISTAMINE EYE DROPSEye Itch Relief
UP TO 12HOURS
Works in Minutes
Original Prescription Strength
For Ages 3 Years And Older
10 DAY SUPPLY
20 [4 pouches x five 0.4 mL] SINGLE-DOSE VIALS
Discard unused portionSTERILE
9677202
622243
-
INGREDIENTS AND APPEARANCE
ALAWAY PRESERVATIVE FREE
ketotifen fumarate solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24208-600 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength KETOTIFEN FUMARATE (UNII: HBD503WORO) (KETOTIFEN - UNII:X49220T18G) KETOTIFEN 0.35 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24208-600-01 4 in 1 CARTON 09/24/2020 1 5 in 1 POUCH 1 0.4 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC:24208-600-99 12 in 1 CARTON 09/24/2020 2 1 in 1 POUCH 2 0.4 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA208158 09/24/2020 Labeler - Bausch & Lomb Incorporated (196603781) Establishment Name Address ID/FEI Business Operations Excelvision 274234566 MANUFACTURE(24208-600)