Label: ARTISS FIBRIN SEALANT- fibrinogen human, human thrombin solution
-
NDC Code(s):
0338-8503-01,
0338-8503-02,
0338-8503-03,
0338-8503-04, view more0338-8503-09, 0338-8503-10, 0338-9637-01, 0338-9639-01, 0338-9641-01
- Packager: Baxter Healthcare Corporation
- Category: PLASMA DERIVATIVE
Drug Label Information
Updated June 9, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ARTISS safely and effectively. See full prescribing information for ARTISS.
ARTISS [Fibrin Sealant (Human)]
Solution for topical application
Initial U.S. Approval: 2008RECENT MAJOR CHANGES
Dosage and Administration (2.1, 2.2) 06/2022
Warnings and Precautions (5.3) 06/2022
INDICATIONS AND USAGE
ARTISS is a fibrin sealant indicated to:
- •
- Adhere autologous skin grafts to surgically prepared wound beds resulting from burns in adult and pediatric populations greater than or equal to 1 year of age (1)
- •
- Adhere tissue flaps during facial rhytidectomy surgery (face-lift) (1)
ARTISS is not indicated as an adjunct to hemostasis (1)
DOSAGE AND ADMINISTRATION
For topical use only. (2)
Individualize the amount based on the size of the surface to be covered. (2)- •
- 2 mL will cover approximately 100 cm2 surface area
- •
- 4 mL will cover approximately 200 cm2 surface area
- •
- 10 mL will cover approximately 500 cm2 surface area
Apply a thin layer using the Cannula or EASYSPRAY and Spray Set or an equivalent device cleared by FDA for application of ARTISS. (2.2)
DOSAGE FORMS AND STRENGTHS
ARTISS solution is available as a 2 mL, 4 mL, and 10 mL (total volume) pre-filled syringe with the DUO Set A (AST Syringe) or DUPLOJECT COMBI (PRIMA Syringe). (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- •
- Hypersensitivity reactions, including anaphylaxis, can occur. Should symptoms occur, discontinue and administer appropriate treatment. (5.1)
- •
- To reduce the risk of potential life-threatening gas embolism, spray using only the appropriate pressurized gas at the recommended pressure (21.8-29.0 psi) and distance (10-15 cm). Use the EASYSPRAY device connected to a Medical grade CO2, Compressed Air or Nitrogen (5.2)
- •
- ARTISS may denature when exposed to solutions containing alcohol, iodine or heavy metals. (5.3)
- •
- As ARTISS is made from human plasma, it may carry the risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) and theoretically, the classic Creutzfeldt-Jakob disease agent (5.4)
ADVERSE REACTIONS
Adverse reactions reported during clinical trials in greater than 1% of subjects were:
Burns: Skin graft failure, hematoma and pruritus (6.1)
Facial Rhytidectomy: Hematoma/seroma (6.1)To report SUSPECTED ADVERSE REACTIONS, contact Baxter Healthcare Corporation at 1-888-229-0001 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Preparation
2.2 Method of Application
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Application Precautions
5.3 Protein Denaturation
5.4 Transmission of Infectious Agents
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
ARTISS is a fibrin sealant indicated to:
- •
- Adhere autologous skin grafts to surgically prepared wound beds resulting from burns in adult and pediatric populations greater than or equal to 1 year of age.
- •
- Adhere tissue flaps during facial rhytidectomy surgery (face-lift).
ARTISS is not indicated as an adjunct to hemostasis.
-
2 DOSAGE AND ADMINISTRATION
For topical use only.
2.1 Preparation
Do not expose to temperatures above 37°C.
Do not microwave.
Do not refrigerate or re-freeze after thawing.
Do not use ARTISS unless it is completely thawed and warmed to 33˚C-37˚C.
Remove the protective syringe cap only after thawing is complete and the application tip is ready to be attached.
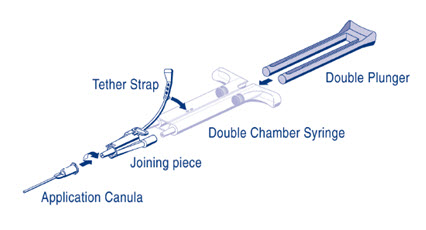
For PRIMA Syringe: To facilitate removal of the tip cap from the syringe, rock the tip cap by moving it backward and forward, then pull the protective cap off the syringe.AST Syringe – Insert the plunger rod into the syringe barrel in the sterile field. (Figure 1)
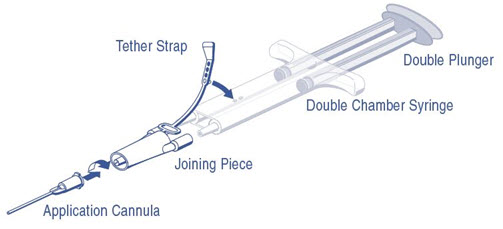
PRIMA Syringe – The plunger is already assembled with the syringe barrel (Figure 2).Room Temperature Thawing
Unopened pouches can be stored for up to 14 days at 15oC to 25°C. Before use, warm the product to 33oC- 37oC.
Table 1 Approximate Room Temperature Thawing and Incubator Warming Times Pack Size in Pouch
Room Temperature
(In Pouches)
15 oC to 25oCWarming thawed pouches
prior to use
33oC to 37oC IncubatorPRIMA Syringe
AST Syringe
PRIMA Syringe
AST Syringe
2 mL
80 minutes
60 minutes
16 minutes
15 minutes
4 mL
90 minutes
110 minutes
21 minutes
25 minutes
10 mL
160 minutes
160 minutes
35 minutes
35 minutes
Quick Thawing
Sterile Water Bath (Quick Thawing): Transfer inner pouch to the sterile field, remove pre-filled syringe from inner pouch and place directly into sterile water bath ensuring the syringe is completely immersed in the water. Maintain the product at 33°C to 37°C until use. Once the package is opened or the product is warmed to 33°C to 37°C, it must be used within 12 hours.
Non-Sterile Water Bath: Maintain the pre-filled syringe in pouches and place into a water bath outside the sterile field ensuring the pouches remain submerged. Remove from the water bath after thawing and warming, dry the external pouch and transfer inner pouch with pre-filled syringe onto the sterile field. Maintain the product at 33°C to 37°C until use. Once the package is opened or the product is warmed to 33°C to 37°C, it must be used within 12 hours.
Incubator: Maintain the pre-filled syringe in pouches and place into an incubator. Remove from the incubator after thawing and warming. Transfer inner pouch with pre-filled syringe onto the sterile field. Maintain the product at 33°C to 37°C until use. Once the package is opened or the product is warmed to 33°C to 37°C, it must be used within 12 hours.
Table 2 Approximate Water Bath or Incubator Thawing and Warming Times Pack Size in Pouch
Sterile Water Bath
(Pouches Removed)
33 oC to 37°CNon-Sterile Water Bath
(In Pouches)
33 oC to 37°CIncubator
(In Pouches)
33 oC to 37°CPRIMA Syringe
AST Syringe
PRIMA Syringe
AST Syringe
PRIMA Syringe
AST Syringe
2 mL
5 minutes
5 minutes
15 minutes
30 minutes
40 minutes
40 minutes
4 mL
5 minutes
5 minutes
20 minutes
40 minutes
50 minutes
85 minutes
10 mL
10 minutes
12 minutes
35 minutes
80 minutes
90 minutes
105 minutes
The sealer protein and the thrombin solutions should be clear or slightly opalescent. Do not use solutions that are cloudy or have deposits. Before use, check the thawed product visually for particles, discoloration or other changes in its appearance. If one of the above occurs, dispose of the solutions.
The thawed sealer protein solution should be liquid but slightly viscous. If the solution has the consistency of a solidified gel, do NOT use ARTISS.
2.2 Method of Application
ARTISS must be applied only to application sites that are visible. Individualize the amount to be applied based on the size of the surface to be covered. Prior to applying ARTISS, dry the site of application using standard techniques (e.g., intermittent application of compresses, swabs, use of suction devices). Do not use pressurized air or gas for drying the site. Apply ARTISS as a thin layer.
The approximate surface areas covered by each package size are:
Table 3 Approximate area requiring skin graft fixation
Required package size of ARTISS
100 cm2
200 cm2500 cm2
2 mL
4 mL
10 mLWhen applying ARTISS using a spray device, utilize the recommended gas, pressure and distance from tissue within the ranges recommended by the manufacturer as follows:
Table 4: Recommended Application Equipment, Gas and Parameters. - *
- Medical grade CO2 is the preferred gas for application, however Compressed Air or Nitrogen are acceptable gasses for administration of ARTISS in open surgery.
Surgery
Spray Set / Applicator tips to use
Pressure regulator
to useGas
Distance
Spray Pressure
Open surgery
TISSEEL/ARTISS
Spray SetEASY SPRAY pressure Regulator
Medical grade
CO2*, Compressed Air or Nitrogen
10-15 cm
1.5-2.0 bar
(21.8-29.0 psi)Application with Spray Device
Apply using the EASYSPRAY and Spray Set, or an equivalent device cleared by FDA for application of ARTISS. See additional instructions for use provided with the EASYSPRAY and Spray Set. Artiss must not be used with the EASYSPRAY / Spray Set system in enclosed body cavities.
Ensure that parts of the body outside the desired application area are sufficiently covered to prevent tissue adherence at undesired site.
Apply as a thin layer to avoid the formation of excess granulation tissue and to ensure gradual absorption of the polymerized fibrin sealant. Excessive clot thickness may delay the natural wound healing process. Ensure that the amount applied is sufficient to entirely cover the intended application area.
The aerosolized sealant should be applied to the wound in a painting motion from side to side to achieve an even thin layer. The wound bed will glisten in the area to which fibrin sealant has been applied. Any areas not covered by fibrin sealant will be clearly visible.
Attach the skin flap or graft to the wound bed immediately after ARTISS has been sprayed. Wet gloves with normal saline before product contact to prevent adherence. The surgeon has up to 60 seconds to manipulate and position the flap or graft prior to polymerization.
Repeat application, if necessary, to any small areas that may not have been previously treated.
Hold the flap or graft in the desired position by gentle compression for at least 3 minutes to ensure ARTISS sets properly and firmly adheres the skin graft or flap to the underlying tissue. The solidified fibrin sealant reaches its final strength in approximately 2 hours after application.
Application with DUO Set A (AST Syringe) and DUPLOJECT COMBI (PRIMA Syringe)
The cannulas included with the DUO Set A and DUPLOJECT COMBI may be used for small wounds or for edges of a skin graft that did not adhere to the wound bed [see Warnings and Precautions (5.3)]. Immediately before application, expel and discard the first several drops from the application cannula to ensure adequate mixing of the Sealer Protein and Thrombin solutions in cases where very small volumes (1-2 drops) are administered.
DUO Set A (AST Syringe) Instructions (see Figure 1):
- 1.
- Insert Plunger into syringe barrel.
- 2.
- Expel all air from the syringe prior to attaching any application device.
- 3.
- Align the joining piece and tether to the side of the syringe with the tether strap hole.
- 4.
- Connect the nozzles of the double chamber ready-to-use syringe to the joining piece, ensuring that they are firmly attached.
- 5.
- Secure the joining piece by fastening the tether strap to the double chamber ready-to-use syringe.
- 6.
- If the tether strap tears, use the spare joining piece provided in the kit.
- 7.
- Do NOT expel the air remaining inside the joining piece.
- 8.
- Attach an application cannula on to the joining piece.
- 9.
- Do NOT expel the air remaining inside the joining piece and inside the application cannula until the start of the actual application because this may clog the application cannula.
DUPLOJECT COMBI (PRIMA Syringe) Instructions (see Figure 2)
- 1.
- The plunger is attached to the syringe barrel and does not need to be inserted.
- 2.
- Expel all air from the syringe prior to attaching any application device.
- 3.
- Align the joining piece and tether to the side of the syringe with the tether strap hole.
- 4.
- Connect the nozzles of the double chamber ready-to-use syringe to the joining piece, ensuring that they are firmly attached.
- 5.
- Secure the joining piece by fastening the tether strap to the double chamber ready-to-use syringe.
- 6.
- If the tether strap tears, use the spare joining piece provided in the kit.
- 7.
- Do NOT expel the air remaining inside the joining piece.
- 8.
- Attach an application cannula on to the joining piece.
- 9.
- Do NOT expel the air remaining inside the joining piece and inside the application cannula until the start of the actual application because this may clog the application cannula.
Note: Interruption of application causes clogging in the cannula. Replace the cannula immediately prior to resuming application. If the opening of the joining piece (Y connector) facing the cannula is clogged, use the spare joining piece provided in the package.
Pre-filled syringes are for single use only. Discard unused contents.
When using application devices cleared by FDA for use with ARTISS, strictly follow the Instruction for Use of the device.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Do not inject directly into the circulatory system or into highly vascularized tissue. Intravascular application can result in life-threatening thromboembolic events and can increase the likelihood and severity of acute hypersensitivity reaction in susceptible patients.
Do not use in individuals with a known hypersensitivity to aprotinin and/or hypersensitivity to any of the active substances or excipients of ARTISS including proteins such as fibrinogen, thrombin, and human albumin [see Warnings and Precautions (5.1) and Adverse Reactions (6)].
Do not spray where the minimum recommended distance from the applicator tip to the target site cannot be assured.
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis, can occur. Cases have been reported in post-marketing experience with fibrin sealant. In specific cases, these reactions have progressed to anaphylaxis. Such reactions may especially be seen if product is applied repeatedly over time or in the same setting, or if systemic aprotinin has been administered previously; however, these reactions may also occur in patients receiving ARTISS for the first time. A first successful treatment does not exclude a later allergic reaction. Symptoms associated with allergic anaphylactic reactions include: Flushing urticaria, pruritus, nausea, drop in blood pressure, tachycardia or bradycardia, dyspnea, severe hypotension and anaphylactic shock.
Aprotinin, a monomeric polypeptide, is known to be associated with anaphylactic reactions. Even in the case of strict local application of aprotinin, there is a risk of anaphylactic reactions to aprotinin, particularly in the case of previous exposure [see Contraindications (4)].
Discontinue administration in the event of hypersensitivity reactions. Remove the already applied, polymerized product from the surgical field. Mild reactions can be managed with antihistamines. Severe reactions and reactions involving hypotension require immediate resuscitative intervention.
5.2 Application Precautions
Any application of pressurized air or gas is associated with a potential risk of air or gas embolism, tissue rupture, or gas entrapment with compression, which may be life threatening or fatal.
Life threatening/fatal air or gas embolism has occurred with the use of spray devices employing pressure regulator to administer fibrin sealants. This can occur if a spray device is used at higher than recommended pressures and in closer than recommended proximity to the tissue surface. The risk appears to be higher when fibrin sealants are sprayed with air, as compared to CO2 and therefore cannot be excluded with ARTISS when sprayed in open surgical procedures.
To reduce the risk of a potentially life-threatening gas embolism when using a spray device, be sure to use the pressure within the pressure range recommended by the spray device manufacturer. In the absence of a specific recommendation avoid using pressure above 20-25 psi. Do not spray closer than the distance recommended by the spray device manufacturer. In the absence of a specific recommendation avoid spraying closer than 10-15 cm from the surface of the tissue. When spraying, changes in blood pressure, pulse, oxygen saturation and end tidal CO2 should be monitored because of the possibility of occurrence of air or gas embolism. When using the EASYSPRAY device, or an equivalent spray device cleared by FDA, use the pressure within the pressure range recommended by the spray device manufacturer. Spray only on to visible application sites, not in enclosed body cavities.
5.3 Protein Denaturation
Solutions containing alcohol, iodine or heavy metals may interfere with the product’s performance due to denaturation of proteins or other mechanisms. If any of these substances have been used to clean the wound area, the area should be thoroughly rinsed and dried before application of ARTISS.
5.4 Transmission of Infectious Agents
ARTISS is made from human plasma. Because this product is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and theoretically, the Creutzfeldt-Jakob disease (CJD) agent. All infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Baxter Healthcare Corporation, at 1-888-229-0001.
Some viruses, such as parvovirus B19, are particularly difficult to remove or inactivate at this time. Parvovirus B19 most seriously affects pregnant women (fetal infection), immune-compromised individuals or individuals with an increased erythropoiesis (e.g., hemolytic anemia) [see Pregnancy (8.1) and Patient Counseling Information (17)].
-
6 ADVERSE REACTIONS
The most frequent (≥ 1% of clinical trial subjects) adverse reactions with the use of ARTISS were: Skin graft failure, hematoma and pruritus in burn studies, and hematoma/seroma in rhytidectomy studies.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The burn and rhytidectomy surgery trials were prospective, randomized, controlled, multicenter clinical trials with a total of 298 subjects. In each trial, the subject served as its own control. All subjects treated have been included into the safety analysis. [see Clinical Studies (14)]
The data described in Table 5 reflects the exposure to ARTISS in the 4 burn and rhytidectomy surgery trials:
Table 5 Trial Population Demographics
Burn
Rhytidectomy
Parameter
Preliminary Trial
Confirmatory Trial
Preliminary Trial
Confirmatory Trial
Sample size (N)
40
138
45
75
Gender
F (%) /
M (%)
11 (27.5%)
29 (72.5%)
44 (31.9%)
94 (68.1%)
42 (93.3%)
3 (6.7%)
71 (94.7%)
4 (5.3%)Age Range (years)
6-55
1-63
43-70
40-71
Volume applied
(Mean ± SD)
(Range in mL)
2.9 ± 1.64
(Range: 1.0-10.8)
2.7 ± 1.9
(Range: 0.2-12.0)
2.32 ± 0.95
(Range: 0.80-4.0)
2.58 ± 1.17
(Range: 0.60-4.0)Adverse reactions in the burn trials occurring in greater than 1% of subjects were skin graft failure (3%), hematoma (1%) and pruritus (1%) [n=178].
Adverse reactions in the facial rhytidectomy trials occurring in greater than 1% of subjects were hematoma/seroma (4%) [n=120].
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post-approval of ARTISS. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to product exposure.
Air embolism [See Application Precautions (5.2)]
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on ARTISS use in pregnant women to inform a drug-associated risk of adverse developmental outcomes. Animal reproduction studies have not been conducted. It is also not known whether ARTISS can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Some viruses, such as parvovirus B19, are particularly difficult to remove or inactivate at this time. Parvovirus B19 most seriously affects pregnant women (fetal infection).The estimated background risk of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There are no direct or controlled studies of ARTISS in lactating women. It is not known whether this drug is excreted in human milk, there is no information regarding the effects on the breastfed infant, and the effects on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ARTISS and any potential adverse effects on the breastfed child from ARTISS or from underlying maternal condition.
8.4 Pediatric Use
In two clinical trials utilizing ARTISS to adhere autologous skin grafts to surgically prepared wound beds resulting from burns, the efficacy and safety in 38 pediatric subjects (27 subjects ages 1-10 years and 11 subjects ages 11-16 years) were not different from an adult population.
-
10 OVERDOSAGE
To avoid the formation of excess granulation tissue and to ensure gradual absorption of the polymerized fibrin sealant, apply only a thin layer [ Dosage and Administration (2.2)].
-
11 DESCRIPTION
ARTISS [Fibrin Sealant (Human)] is a two-component fibrin sealant made from pooled human plasma.
Sealer Protein Solution
Total protein: 96 -125 mg/mL
Fibrinogen: 67 - 106 mg/mL
Fibrinolysis Inhibitor (Synthetic): 2250 - 3750 Kallidinogenase Inhibiting Unit/mLOther ingredients include: Human albumin, tri-sodium citrate, histidine, niacinamide, polysorbate 80 and water for injection (WFI).
Thrombin Solution
Thrombin (Human): 2.5 - 6.5 units/mL1
Calcium Chloride: 36 - 44 µmol/mL
Other ingredients include: Human albumin, sodium chloride and water for injection (WFI).1 The potency expressed in units is determined using a clotting assay against an internal reference standard for potency that has been calibrated against the World Health Organization (WHO) Second International Standard for Thrombin, 01/580. Therefore, a unit is equivalent to an International Unit (IU).
Sealer Protein (Human)
Sealer Protein (Human) is a sterile, non-pyrogenic, vapor-heated and solvent/detergent treated preparation made from pooled human plasma. Sealer Protein (Human) is provided as a frozen liquid solution pre-filled into one side of a dual-chambered syringe (1). The active ingredient in Sealer Protein (Human) is fibrinogen. A Fibrinolysis Inhibitor, Aprotinin (Synthetic) is included in the Sealer Protein (Human) component to delay fibrinolysis. Aprotinin (Synthetic) is manufactured by solid phase synthesis from materials completely of non-human/non-animal origin.
Thrombin (Human)
Thrombin (Human) is a sterile, non-pyrogenic, vapor-heated and solvent/detergent treated preparation made from pooled human plasma. Thrombin (Human) is provided as a frozen liquid solution pre-filled into one side of a dual-chambered syringe (2).
Sealer Protein (Human) and Thrombin (Human) are made from pooled human plasma collected at US licensed collection centers. The vapor heat and solvent/detergent treatment steps used in the manufacturing process have been shown to be capable of significant viral reduction. No procedure, however, has been shown to be completely effective in removing viral infectivity from derivatives of human plasma [see Viral Clearance below and Warnings and Precautions (5.4)].
Viral Clearance
The manufacturing procedure includes processing steps designed to further reduce the risk of viral transmission. In particular, vapor heating and solvent/detergent treatment processes are included in the manufacturing of Sealer Protein Concentrate and Thrombin. Validation studies were conducted using samples drawn from manufacturing intermediates for each of the two human plasma derived components. These samples were spiked with stock virus suspensions of known titers followed by further processing under conditions equivalent to those in the respective manufacturing steps. The stock virus suspensions represent HIV, HBV, HCV, HAV and Human Parvovirus B19.
The virus reduction factors (expressed as log10) of independent manufacturing steps are shown in Table 6 for each of the viruses tested:
Table 6 Reduction Factors for Virus Removal and/or Inactivation
Sealer Protein ComponentManufacturing Step
Mean Reduction Factors [log10] of Virus Tested
HIV-1
HAV
BVDV
PRV
MMV
Early Manufacturing Steps
n.d.
n.d.
n.d.
n.d.
2.7
Solvent/Detergent Treatment
>5.3
n.d.
>5.7
>5.9
n.d.
Vapor Heat Treatment
>5.5
>5.6
>5.7
>6.7
1.2
Overall Reduction Factor (ORF)
>10.8
>5.6
>11.4
>12.6
3.9
Reduction Factors for Virus Removal and/or Inactivation
Thrombin ComponentMean Reduction Factors [log10] of Virus Tested
Manufacturing Step
HIV-1
HAV
BVDV
PRV
MMV
Thrombin precursor mass capture
3.2
1.5
1.8
2.5
1.2
Vapor Heat Treatment
>5.5
>4.9
>5.3
>6.7
1.0
Solvent/Detergent Treatment
>5.3
n.d.
>5.5
>6.4
n.d.
Ion Exchange Chromatography
n.d.
n.d.
n.d.
n.d.
3.6
Overall Reduction Factor (ORF)
>14.0
>6.4
>12.6
>15.6
5.8
n.d. = not determined
HIV-1: Human immunodeficiency virus 1; HAV: Hepatitis A virus; BVDV: Bovine viral diarrhea virus, a model for Hepatitis C virus; PRV: Pseudorabies virus, a model for enveloped DNA viruses, among those Hepatitis B virus; MMV: Mice minute virus, a model for B19V.
In addition, Human Parvovirus B19 was used to investigate the upstream Thrombin precursor mass capture step, the Sealer Protein early manufacturing steps and the Thrombin and Sealer Protein vapor heating steps. Using quantitative PCR assays, the estimated log reduction factors obtained were 1.7 and 3.4 for the Thrombin precursor mass capture step and Sealer Protein early manufacturing steps and >4 / 1.0 for the Thrombin / Sealer Protein vapor heating steps, respectively.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Upon mixing Sealer Protein (Human) and Thrombin (Human), the two components mimic the final stage of the blood coagulation cascade. Soluble fibrinogen is transformed into fibrin that adheres to the wound surface and to the skin flap or graft to be affixed. Due to the low thrombin concentration, initial polymerization will take up to 60 seconds. The fibrin clot continues to strengthen for up to 2 hours after application.
Spray application over the wound bed provides full surface adherence of skin flaps and grafts. Full surface adherence minimizes areas of dead space between the wound bed and applied tissues. Elimination of dead space prevents shear irritation upon movement as well as reduces the void space under the skin that can host fluid build-up.
12.2 Pharmacodynamics
Thrombin is a highly specific protease that transforms the fibrinogen contained in Sealer Protein (Human) into fibrin.
Fibrinolysis Inhibitor, Aprotinin (Synthetic), is a polyvalent protease inhibitor that prevents premature degradation of fibrin. Free Aprotinin and its metabolites have a half-life of 30 to 60 minutes and are eliminated by the kidney. Preclinical studies with different fibrin sealant preparations simulating the fibrinolytic activity generated by extracorporeal circulation in patients during cardiovascular surgery have shown that incorporation of aprotinin in the product formulation increases resistance of the fibrin sealant clot to degradation in a fibrinolytic environment.
-
14 CLINICAL STUDIES
Burns (grafts)
ARTISS was investigated for adherence of split thickness sheet skin grafts in burn patients in a prospective, randomized, controlled, evaluator-blinded, multicenter clinical trials. In each of the 138 patients, two comparable test sites were identified after burn wound excision. Skin grafts were adhered at one test site using ARTISS, and at the other test site using staples (control). The study product was applied once to the wound bed of the allocated test site during skin grafting surgery.
The mean ± standard deviation (SD) estimated total body surface area (TBSA) for all burn wounds was 13.6 ± 9.2%. The mean ± SD estimated TBSA requiring skin grafting was 8.0 ± 6.9%. The mean ± SD estimated TBSA for ARTISS test sites was 1.7 ± 0.8% and for the stapled test sites was 1.7 ± 0.7%. Burn wound thickness was classified as full thickness in 106 (76.8%) of the 138 treated subjects, and partial thickness in 32 (23.2%) subjects.
The safety population contained all 138 treated subjects; however, 11 subjects did not have an available primary endpoint assessment, leaving a modified intent-to-treat (ITT) set of 127 patients. Complete wound closure by Day 28 was achieved in 43.3% of the ARTISS test sites and 37.0% of the stapled test sites in the 127 ITT patients. Wound closure at Day 28 was complete at 72% of the ARTISS and staples test sites for the 1-6 years old group (N=18), at 32% of the ARTISS test sites and 26% of the staples test sites for the 7-18 years old group (N=19) and at 40% of the ARTISS test sites and 32% of the staples test sites for the greater then 18 years old group [ITT]. The lower limit of the 97.5% confidence interval of the difference between ARTISS and staples was -0.029. A similar result was obtained in the per protocol (PP) population: complete wound closure by Day 28 was achieved in 45.3% of the ARTISS test sites and 39.6% of the stapled test sites in the 106 PP patients. The lower limit of the 97.5% confidence interval of the difference between ARTISS and staples was -0.041. Therefore, ARTISS was found to be non-inferior to staples in the ITT and PP populations at the 97.5% one-sided level for complete wound closure by Day 28 because the lower limit of the confidence interval of the difference between ARTISS and staples success rates was greater than the predefined limit of -0.1.
Facial Rhytidectomy (flaps)
ARTISS was investigated for adherence of skin flaps in facial rhytidectomy surgeries during two prospective, randomized, controlled, multicenter clinical trials. Both the preliminary trial investigating 45 subjects and the confirmatory trial with 75 subjects had a split-face design in which 1 side of the face was treated with ARTISS as an adjunct to the standard of care (SoC) and the other side received SoC only, which was closure of the flap by means of staples and suturing only; therefore each subject participated in both arms (ARTISS and SoC).
Primary endpoint of the confirmatory trial conducted in 75 subjects was the total drainage volume collected from each side of the face at 24 h (±4 h) post surgery. Occurrence of hematoma and seroma on each side of the face, comparison of edema between the 2 sides of the face, changes in skin sensitivity from baseline on each side of the face and subject preference were evaluated as secondary endpoints.
In both trials, a standardized drain was placed in each side of the face prior to the flap closure and drainage volume from both sides of the face from all subjects was compared. Pressure dressings were not allowed.
The results for the primary endpoint of the confirmatory trial are presented in Table 7a below.
Table 7a Drainage Volume Comparison at 24 h Post Operative in Confirmatory Trial Clinical Trial (n=75)
Mean ± SD Drainage (mL) ARTISS Side of the Face
Mean ± SD Drainage (mL) SoC Side of the Face
p-Value
Confirmatory trial
7.7 ± 7.4
20.0 ± 11.3
<0.0001
A statistically significant difference in drainage volumes was observed, favoring the side of the face treated with ARTISS.
Drainage volumes at 24 h post operatively for each side of the face reported as secondary endpoint in the preliminary trial are presented in Table 7b below.
Table 7b
Drainage Volume Comparison at 24 h Post Operative in Preliminary Trial
Clinical Trial (n=45)
Mean ± SD Drainage (mL) ARTISS Side of the Face
Mean ± SD Drainage (mL) SoC Side of the Face
Preliminary trial
11.5 ± 13.7
26.8 ± 24.0
An integrated analysis of the occurrence of hematoma/seroma in all 120 subjects across two trials was performed. A comparison of the proportion of subjects experiencing a hematoma/seroma exclusively on the ARTISS-treated side or on the SoC side of the face is presented in Table 8 below.
Table 8 Occurrence of Hematoma/Seroma Clinical Trial
ARTISS
n (%)SoC
n (%)Both Sides of Face
n (%)Total
n (%)Preliminary trial
0
9 (20%)
0
9 (20%)
Confirmatory trial
2 (2.7%)
5 (6.7%)
3 (4%)
10 (13.3%)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Table 9 (AST Syringe) Pack Size
Packaging Component
NDC
2 mL
Carton
0338-8503-02
Pouch
0338-8503-01
4 mL
Carton
0338-8503-04
Pouch
0338-8503-03
10 mL
Carton
0338-8503-10
Pouch
0338-8503-09
Table 10 (PRIMA Syringe) Pack Size
Packaging Component
NDC
2 mL
Carton
0338-9637-01
4 mL
Carton
0338-9639-01
10 mL
Carton
0338-9641-01
Storage
Store in original carton to protect from light. Do not use after the expiration date. Discard if packaging of any component is damaged.
- •
- Long term: Store at ≤ -20°C.
- •
- Short term: Room Temperature Thawing: Unopened pouches, thawed at room temperature, may be stored for up to 14 days at room temperature (15°C to 25°C) after removal from the freezer.
Quick Thawing: Maintain the product at 33°C to 37oC until use. If the product is removed from original pouch or warmed to 33°C to 37oC it must be used within 12 hours. - Do not refrigerate or re-freeze after thawing. Do not microwave.
-
17 PATIENT COUNSELING INFORMATION
Inform patients that ARTISS is made from human plasma and discuss the risks and benefits with the patient.
Parvovirus B19 infection may be serious for pregnant women (fetal infection) and for individuals with immunodeficiency or increased red blood cell turnover. Instruct patients to consult their physician if symptoms of B19 virus infection appear (fever, drowsiness, chills and runny nose followed about two weeks later by a rash and joint pain) [see Pregnancy (8.1)].
Manufactured For Baxter Healthcare Corporation
Deerfield, IL 60015 USA
US License No. 140
Baxter, Artiss, Duploject, Easyspray and Tisseel are trademarks of Baxter International Inc.
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Fibrin Sealant (Human)
ARTISS 2 mLNDC 0338-8503-01
Frozen
Baxter Logo
Temperature sensitive – Do NOT expose above 37°C (99°F).
Contents:
Pre-filled syringe containing:
– Sealer Protein Solution ➊: 1 mL, sterile
– Sealer Protein (Human)
– Fibrinolysis Inhibitor (Aprotinin,
Synthetic), 3000 KIU/mL
– Thrombin Solution ➋: 1 mL, sterile
– Thrombin (Human), 4 units/mL
– Calcium Chloride, 40 μmol/mLTOPICAL USE ONLY
DO NOT INJECT
Read directions for thawing and application before use.
Store at –20°C (–4°F) or colder. Unopened pouches,
thawed at room temperature, may be stored for up to
14 days at 15 – 25°C.
Do not refrigerate, microwave, or re-freeze.
Rx Only
Contents were sterilized and
packaged under aseptic conditions.Manufactured for Baxter
Healthcare Corporation
Deerfield IL, 60015 USA
U.S. License No. 140
0752634Lot No.:
Exp. Date:
0752635
Fibrin Sealant (Human)
ARTISSTOPICAL USE ONLY
NDC 0338-8503-02
2 mL
Pre-filled SyringeBarcode
N3 0338-8503-02 7Fibrin Sealant (Human)
ARTISSTOPICAL USE ONLY
2 mL
Pre-filled SyringeContents:
Pre-filled syringe containing:Sealer Protein Solution: 1 mL, sterile
- •
- Sealer Protein (Human), 86.5 mg/Ml
Vapor Heated, Solvent/Detergent Treated
- •
- Fibrinolysis Inhibitor (Aprotinin, Synthetic), 30000 KIU/Ml
Thrombin Solution: 1Ml, sterile
- •
- Thrombin (Human), 4 units/Ml
Vapor Heated, Solvent/Detergent Treated
- •
- Calcium Chloride, 40 µmol/Ml
TOPICAL USE ONLY
DO NOT INJECTDo not use on individuals with a known hypersensitivity to aprotinin
and/or any of the active substances or excipients.The risks and benefits of this product should be discussed with the
patient.Read full prescribing information before use.
Store at –20°C (–4°F) or colder.
Unopened pouches, thawed at room temperature, may be stored for
up to 14 days at 15 – 25°C.Do not refrigerate, microwave, or re-freeze.
For single use only
No preservative
Not made with natural rubber latex
Rx only
Baxter Logo
Fibrin Sealant (Human)
ARTISSTOPICAL USE ONLY
2 Ml
Pre-filled SyringeFibrin Sealant (Human)
ARTISSTOPICAL USE ONLY
736602
Fibrin Sealant (Human)
ARTISSTOPICAL USE ONLY
2 Ml
Pre-filled Syringe2 Ml
Pre-filled SyringeNDC 0338-8503-02
Fibrin Sealant (Human)
ARTISSTOPICAL USE ONLY
2 Ml
Pre-filled SyringeVapor Heated, Solvent/Detergent Treated, Frozen
TOPICAL USE ONLY
DO NOT INJECTManufactured for Baxter Healthcare Corporation
Deerfield, IL 60015 USA
1-888-229-0001U.S. License No. 140
Made in AustriaReorder Number: 1501651
Baxter and Artiss are trademarks of Baxter
International Inc.Baxter Logo
GTIN (01) 00303388503027
LOT (01)
EXPIRY (17)
SERIAL (21)Baxter Logo
DUO Set A 2 ml / 4 ml
Indications for Use:
For the application of ARTISS Fibrin SealantFor single use only. Not made with natural rubber latex.
Do not reuse or resterilize. Sterilized by gamma radiation.
Sterile and non-pyrogenic in unopened and undamaged package.
Rx Only.Manufactured for:
Baxter Healthcare Corporation
One Baxter Parkway
Deerfield, IL 60015 USAMade in Belgium
Lot No.
Exp. Date:REF
BE-90-01-042
Fibrin Sealant (Human)
ARTISS 2 mL
NDC 0338-9637-01
FrozenBaxter Logo
Temperature sensitive – Do NOT expose above 37°C (99°F).
Contents:
Pre-filled syringe containing:
– Sealer Protein Solution ➊: 1 mL, sterile
– Fibrinogen (Human), 86.5 mg/mL
– Fibrinolysis Inhibitor (Aprotinin,
Synthetic), 3000 KIU/mL
– Thrombin Solution ➋: 1 mL, sterile
– Thrombin (Human), 4 units/mL
– Calcium Chloride, 40 μmol/mLTOPICAL USE ONLY
DO NOT INJECT
Read directions for thawing and application before use.
Store at –20°C (–4°F) or colder. Unopened pouches,
thawed at room temperature, may be stored for up to
14 days at 15 – 25°C.
Do not refrigerate, microwave, or re-freeze.
Rx Only
Contents were sterilized and
packaged under aseptic conditions.Manufactured for Baxter
Healthcare Corporation
Deerfield IL, 60015 USA
U.S. License No. 140
0752951Barcode
Green triangle symbol
Barcode
Lot No.:
Exp. Date:
Baxter Logo
Artiss 2 mL
Fibrin Sealant (Human)
Vapor Heated, Solvent/Detergent Treated, FrozenNDC 0338-9637-01
2 mLTOPICAL USE ONLY
DO NOT INJECTManufactured for Baxter Healthcare
Corporation
Deerfield, IL 60015 USA
1-888-229-0001U.S. License No. 140
Made in AustriaReorder Number: 5500694
Baxter and Artiss are trademarks of Baxter
International Inc.GTIN (01) 00303389637011
LOT (10)
EXPIRY (17)
SERIAL (21)Baxter Logo
2 mLBarcode
Artiss 2 mL
Fibrin Sealant (Human)
Vapor Heated, Solvent/Detergent Treated, FrozenBaxter Logo
2 mLBaxter Logo
NDC 0338-9637-01
Artiss 2 mL
Fibrin Sealant (Human)
Vapor Heated, Solvent/Detergent Treated, FrozenContents:
Pre-filled syringe containing:
Sealer Protein Solution: 1 mL, sterile- •
- Fibrinogen (Human), 86.5 mg/mL
Vapor Heated, Solvent/Detergent Treated - •
- Fibrinolysis Inhibitor (Aprotinin, Synthetic), 3000 KIU/mL
Thrombin Solution: 1 mL, sterile
- •
- Thrombin (Human), 4 units/mL
Vapor Heated, Solvent/Detergent Treated - •
- Calcium Chloride, 40 μmol/mL
TOPICAL USE ONLY
DO NOT INJECT
Do not use on individuals with a known hypersensitivity
to aprotinin and/or any of the active substances or
excipients.
The risks and benefits of this product should be
discussed with the patient.
Read full prescribing information before use.
Store at –20°C (–4°F) or colder.
Unopened pouches,thawed at room temperature,
may be stored for up to 14 days at 15 – 25°C.
Do not refrigerate, microwave, or re-freeze.
For Single Patient Use Only
No preservative
Not made with natural rubber latex
Rx only2 mL
Artiss 2 mL
Fibrin Sealant (Human)
Vapor Heated, Solvent/Detergent Treated, Frozen2 mL
0752952
Artiss 2 mL
Fibrin Sealant (Human)
Vapor Heated, Solvent/Detergent Treated, FrozenBarcode
NDC 0338-9637-01
Baxter Logo
2 mL
Barcode
N3 0338-9637-01 1Fibrin Sealant (Human)
ARTISS 4 mL
NDC 0338-9639-01
FrozenBaxter Logo
Temperature sensitive – Do NOT expose above 37°C (99°F).
Contents:
Pre-filled syringe containing:
– Sealer Protein Solution ➊: 2 mL, sterile
– Fibrinogen (Human), 86.5 mg/mL
– Fibrinolysis Inhibitor (Aprotinin,
Synthetic), 3000 KIU/mL
– Thrombin Solution ➋: 2 mL, sterile
– Thrombin (Human), 4 units/mL
– Calcium Chloride, 40 μmol/mLTOPICAL USE ONLY
DO NOT INJECT
Read directions for thawing and application before use.
Store at –20°C (–4°F) or colder. Unopened pouches,
thawed at room temperature, may be stored for up to
14 days at 15 – 25°C.
Do not refrigerate, microwave, or re-freeze.
Rx Only
Contents were sterilized and
packaged under aseptic conditions.Manufactured for Baxter
Healthcare Corporation
Deerfield IL, 60015 USA
U.S. License No. 140
0752955Barcode
Red triangle symbol
Barcode
Lot No.:
Exp. Date:
Baxter Logo
Artiss 4 mL
Fibrin Sealant (Human)
Vapor Heated, Solvent/Detergent Treated, FrozenNDC 0338-9639-01
4 mLTOPICAL USE ONLY
DO NOT INJECTManufactured for Baxter Healthcare
Corporation
Deerfield, IL 60015 USA
1-888-229-0001U.S. License No. 140
Made in AustriaReorder Number: 5500695
Baxter and Artiss are trademarks of Baxter
International Inc.GTIN (01) 00303389639015
LOT (10)
EXPIRY (17)
SERIAL (21)Baxter Logo
4 mLBarcode
Artiss 4 mL
Fibrin Sealant (Human)
Vapor Heated, Solvent/Detergent Treated, FrozenBaxter Logo
4 mLBaxter Logo
NDC 0338-9639-01
Artiss 4 mL
Fibrin Sealant (Human)
Vapor Heated, Solvent/Detergent Treated, FrozenContents:
Pre-filled syringe containing:
Sealer Protein Solution: 2 mL, sterile- •
- Fibrinogen (Human), 86.5 mg/mL
Vapor Heated, Solvent/Detergent Treated - •
- Fibrinolysis Inhibitor (Aprotinin, Synthetic), 3000 KIU/mL
Thrombin Solution: 2 mL, sterile
- •
- Thrombin (Human), 4 units/mL
Vapor Heated, Solvent/Detergent Treated - •
- Calcium Chloride, 40 μmol/mL
TOPICAL USE ONLY
DO NOT INJECT
Do not use on individuals with a known hypersensitivity
to aprotinin and/or any of the active substances or
excipients.
The risks and benefits of this product should be
discussed with the patient.
Read full prescribing information before use.
Store at –20°C (–4°F) or colder.
Unopened pouches,thawed at room temperature,
may be stored for up to 14 days at 15 – 25°C.
Do not refrigerate, microwave, or re-freeze.
For Single Patient Use Only
No preservative
Not made with natural rubber latex
Rx only4 mL
Artiss 4 mL
Fibrin Sealant (Human)
Vapor Heated, Solvent/Detergent Treated, Frozen4 mL
0752956
Artiss 4 mL
Fibrin Sealant (Human)
Vapor Heated, Solvent/Detergent Treated, FrozenBarcode
NDC 0338-9639-01
Baxter Logo
4 mL
Barcode
N3 0338-9639-01 5Fibrin Sealant (Human)
ARTISS 10 mL
NDC 0338-9641-01
FrozenBaxter Logo
Temperature sensitive – Do NOT expose above 37°C (99°F).
Contents:
Pre-filled syringe containing:
– Sealer Protein Solution ➊: 5 mL, sterile
– Fibrinogen (Human), 86.5 mg/mL
– Fibrinolysis Inhibitor (Aprotinin,
Synthetic), 3000 KIU/mL
– Thrombin Solution ➋: 5 mL, sterile
– Thrombin (Human), 4 units/mL
– Calcium Chloride, 40 μmol/mLTOPICAL USE ONLY
DO NOT INJECT
Read directions for thawing and application before use.
Store at –20°C (–4°F) or colder. Unopened pouches,
thawed at room temperature, may be stored for up to
14 days at 15 – 25°C.
Do not refrigerate, microwave, or re-freeze.
Rx Only
Contents were sterilized and
packaged under aseptic conditions.Manufactured for Baxter
Healthcare Corporation
Deerfield IL, 60015 USA
U.S. License No. 140
0752959Barcode
Blue triangle symbol
Barcode
Lot No.:
Exp. Date:
Baxter Logo
Artiss 10 mL
Fibrin Sealant (Human)
Vapor Heated, Solvent/Detergent Treated, FrozenNDC 0338-9641-01
10 mLTOPICAL USE ONLY
DO NOT INJECTManufactured for Baxter Healthcare
Corporation
Deerfield, IL 60015 USA
1-888-229-0001U.S. License No. 140
Made in AustriaReorder Number: 5500696
Baxter and Artiss are trademarks of Baxter
International Inc.GTIN (01) 00303389641018
LOT (10)
EXPIRY (17)
SERIAL (21)Baxter Logo
10 mLBarcode
Artiss 10 mL
Fibrin Sealant (Human)
Vapor Heated, Solvent/Detergent Treated, FrozenBaxter Logo
10 mLBaxter Logo
NDC 0338-9641-01
Artiss 10 mL
Fibrin Sealant (Human)
Vapor Heated, Solvent/Detergent Treated, FrozenContents:
Pre-filled syringe containing:
Sealer Protein Solution: 5 mL, sterile- •
- Fibrinogen (Human), 86.5 mg/mL
Vapor Heated, Solvent/Detergent Treated - •
- Fibrinolysis Inhibitor (Aprotinin, Synthetic),
3000 KIU/mL
Thrombin Solution: 5 mL, sterile
- •
- Thrombin (Human), 4 units/mL
Vapor Heated, Solvent/Detergent Treated - •
- Calcium Chloride, 40 μmol/mL
TOPICAL USE ONLY
DO NOT INJECT
Do not use on individuals with a known hypersensitivity
to aprotinin and/or any of the active substances or
excipients.
The risks and benefits of this product should be
discussed with the patient.
Read full prescribing information before use.
Store at –20°C (–4°F) or colder.
Unopened pouches,thawed at room temperature,
may be stored for up to 14 days at 15 – 25°C.
Do not refrigerate, microwave, or re-freeze.
For Single Patient Use Only
No preservative
Not made with natural rubber latex
Rx only10 mL
Artiss 10 mL
Fibrin Sealant (Human)
Vapor Heated, Solvent/Detergent Treated, Frozen10 mL
0752960
Artiss 10 mL
Fibrin Sealant (Human)
Vapor Heated, Solvent/Detergent Treated, FrozenBarcode
NDC 0338-9641-01
Baxter Logo
10 mL
Barcode
N3 0338-9641-01 8Baxter Logo
DUPLOJECT COMBI
Indication for Use:
For the application of TISSEEL and ARTISS Fibrin Sealant.REF Symbol
Batch code
Use-by dateFor single use only. Not made with natural rubber latex.
Do not reuse or resterilize. Sterilize using ethylene oxide.
Sterile and non-pyrogenic in unopened and undamaged package. Rx Only.Manufactured for:
Baxter Healthcare Corporation
One Baxter Parkway
Deerfield, IL 60015 USA
1-888-229-0001Made in Belguim
BE-90-01-057
-
INGREDIENTS AND APPEARANCE
ARTISS FIBRIN SEALANT
fibrinogen human, human thrombin solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0338-8503 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FIBRINOGEN HUMAN (UNII: N94833051K) (FIBRINOGEN HUMAN - UNII:N94833051K) FIBRINOGEN HUMAN 86.5 mg in 1 mL HUMAN THROMBIN (UNII: 6K15ABL77G) (HUMAN THROMBIN - UNII:6K15ABL77G) HUMAN THROMBIN 4 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength APROTININ (UNII: 04XPW8C0FL) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) ALBUMIN HUMAN (UNII: ZIF514RVZR) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) HISTIDINE (UNII: 4QD397987E) NIACINAMIDE (UNII: 25X51I8RD4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-8503-02 1 in 1 CARTON 1 NDC:0338-8503-01 2 mL in 1 SYRINGE, PLASTIC; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 2 NDC:0338-8503-04 1 in 1 CARTON 2 NDC:0338-8503-03 4 mL in 1 SYRINGE, PLASTIC; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 3 NDC:0338-8503-10 1 in 1 CARTON 3 NDC:0338-8503-09 10 mL in 1 SYRINGE, PLASTIC; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125266 03/19/2008 ARTISS FIBRIN SEALANT
fibrinogen human, human thrombin solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0338-9637 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FIBRINOGEN HUMAN (UNII: N94833051K) (FIBRINOGEN HUMAN - UNII:N94833051K) FIBRINOGEN HUMAN 86.5 mg in 1 mL HUMAN THROMBIN (UNII: 6K15ABL77G) (HUMAN THROMBIN - UNII:6K15ABL77G) HUMAN THROMBIN 4 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength APROTININ (UNII: 04XPW8C0FL) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) ALBUMIN HUMAN (UNII: ZIF514RVZR) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) HISTIDINE (UNII: 4QD397987E) NIACINAMIDE (UNII: 25X51I8RD4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-9637-01 2 mL in 1 SYRINGE, PLASTIC; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125266 03/19/2008 ARTISS FIBRIN SEALANT
fibrinogen human, human thrombin solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0338-9639 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FIBRINOGEN HUMAN (UNII: N94833051K) (FIBRINOGEN HUMAN - UNII:N94833051K) FIBRINOGEN HUMAN 86.5 mg in 1 mL HUMAN THROMBIN (UNII: 6K15ABL77G) (HUMAN THROMBIN - UNII:6K15ABL77G) HUMAN THROMBIN 4 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength APROTININ (UNII: 04XPW8C0FL) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) ALBUMIN HUMAN (UNII: ZIF514RVZR) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) HISTIDINE (UNII: 4QD397987E) NIACINAMIDE (UNII: 25X51I8RD4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-9639-01 4 mL in 1 SYRINGE, PLASTIC; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125266 03/19/2008 ARTISS FIBRIN SEALANT
fibrinogen human, human thrombin solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0338-9641 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FIBRINOGEN HUMAN (UNII: N94833051K) (FIBRINOGEN HUMAN - UNII:N94833051K) FIBRINOGEN HUMAN 86.5 mg in 1 mL HUMAN THROMBIN (UNII: 6K15ABL77G) (HUMAN THROMBIN - UNII:6K15ABL77G) HUMAN THROMBIN 4 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength APROTININ (UNII: 04XPW8C0FL) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) ALBUMIN HUMAN (UNII: ZIF514RVZR) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) HISTIDINE (UNII: 4QD397987E) NIACINAMIDE (UNII: 25X51I8RD4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-9641-01 10 mL in 1 SYRINGE, PLASTIC; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125266 03/19/2008 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Takeda Manufacturing Austria AG 300466733 ANALYSIS(0338-8503, 0338-9637, 0338-9639, 0338-9641)