Label: DYSPORT- botulinum toxin type a injection, powder, lyophilized, for solution
- NDC Code(s): 15054-0500-1, 15054-0500-2, 15054-0500-9, 15054-0530-6
- Packager: Ipsen Biopharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated September 28, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DYSPORT safely and effectively. See full prescribing information for DYSPORT.
DYSPORT® (abobotulinumtoxinA) for injection, for intramuscular use
Initial U.S. Approval: 2009WARNING: DISTANT SPREAD OF TOXIN EFFECT
See full prescribing information for complete boxed warning
The effects of DYSPORT and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life-threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can occur in adults, particularly in those patients who have underlying conditions that would predispose them to these symptoms (5.1).
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
DYSPORT is an acetylcholine release inhibitor and a neuromuscular blocking agent indicated for:
DOSAGE AND ADMINISTRATION
Preparation of DYSPORT Solution for Administration (2.2)
- Once reconstituted, store in original container in a refrigerator at 2°C to 8°C (36°F to 46°F) and use within 24 hours
- Do not freeze after reconstitution
- Reconstitution instructions are specific for the 300 Unit and 500 Unit vials
- Reconstituted DYSPORT is intended for intramuscular injection only. After reconstitution, DYSPORT should be used for only one injection session and for only one patient
Cervical Dystonia (2.3)
- Initial dose is 500 Units given intramuscularly as a divided dose among the affected muscles
- Re-treatment every 12 to 16 weeks or longer, as necessary, based on return of clinical symptoms with doses administered between 250 Units and 1000 Units to optimize clinical benefit
- Re-treatment should not occur in intervals of less than 12 weeks
- Titrate in 250 Unit steps according to patient's response
Glabellar Lines (2.4)
- Administer a total dose of 50 Units, divided in five equal aliquots of 10 Units each, intramuscularly to affected muscles to achieve clinical effect
- Re-treatment should be administered no more frequently than every 3 months
Spasticity in Adults (2.5)
- Select dose based on muscles affected, severity of spasticity, and treatment and adverse reaction history with botulinum toxins
- Dosing for upper limb spasticity: between 500 Units and 1000 Units
- Dosing for lower limb spasticity: up to 1500 Units
- The maximum recommended total dose per treatment session (upper and lower limb combined) in adults is 1500 Units
- Re-treatment, based on return of clinical symptoms, should not occur in intervals of less than 12 weeks
Spasticity in Pediatric Patients (2.6)
- Select dose based on the affected muscle, severity of spasticity, and treatment and adverse reaction history with all botulinum toxins.
- Recommended dosing for upper limb spasticity: 8 Units/kg to 16 Units/kg per limb. The maximum recommended total dose administered per treatment session must not exceed 16 Units/kg or 640 Units, whichever is lower.
- Recommended dosing for lower limb spasticity: 10 Units/kg to 15 Units/kg per limb. Total dose per treatment session must not exceed 15 Units/kg for unilateral lower limb injections, 30 Units/kg for bilateral injections, or 1000 Units, whichever is lower.
- The maximum recommended total dose per treatment session is 30 Units/kg or 1000 Units, whichever is lower. Re-treatment, based on return of clinical symptoms, should not occur in intervals of less than 3 months.
DOSAGE FORMS AND STRENGTHS
- For Injection: 300 Units or 500 Units lyophilized powder in a single-dose vial (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- The potency units of DYSPORT are not interchangeable with other preparations of botulinum toxin products (5.2)
- Immediate medical attention may be required in cases of respiratory, speech or swallowing difficulties (5.4)
- Recommended dose and frequency of administration should not be exceeded (5.5)
- Dry eye may occur with glabellar line treatment; if symptoms persist, consider referring patient to an ophthalmologist (5.6)
- Concomitant neuromuscular disorder may exacerbate clinical effects of treatment (5.7)
ADVERSE REACTIONS
Most commonly observed adverse reactions are (6.1):
Cervical Dystonia
(≥5%): muscular weakness, dysphagia, dry mouth, injection site discomfort, fatigue, headache, musculoskeletal pain, dysphonia, injection site pain and eye disorders
Glabellar Lines
(≥2%): nasopharyngitis, headache, injection site pain, injection site reaction, upper respiratory tract infection, eyelid edema, eyelid ptosis, sinusitis, nausea, and blood present in urine
Spasticity in Adults
- Upper limb spasticity (≥4%): muscular weakness
- Lower limb spasticity (≥5%): falls, muscular weakness, and pain in extremity
Spasticity in Pediatric Patients
- Upper limb spasticity (≥10%): upper respiratory tract infection and pharyngitis
- Lower limb spasticity (≥10%): nasopharyngitis, cough, and pyrexia
To report SUSPECTED ADVERSE REACTIONS, contact Ipsen Biopharmaceuticals, Inc. at 855-463-5127 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
- Concomitant use of DYSPORT and aminoglycosides or other agents interfering with neuromuscular transmission or muscle relaxants, should be observed closely because effect of DYSPORT may be potentiated (7.1, 7.4)
- Anticholinergic drugs may potentiate systemic anticholinergic effects (7.2)
- The effect of administering different botulinum neurotoxins during the course of treatment with DYSPORT is unknown (7.3)
USE IN SPECIFIC POPULATIONS
- Administer DYSPORT with care in elderly patients, reflecting the greater frequency of concomitant disease and other drug therapy (8.5)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 9/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: DISTANT SPREAD OF TOXIN EFFECT
1 INDICATIONS AND USAGE
1.1 Cervical Dystonia
1.2 Glabellar Lines
1.3 Spasticity
2 DOSAGE AND ADMINISTRATION
2.1 Instructions for Safe Use
2.2 Preparation of DYSPORT Solution for Administration
2.3 Dosing in Cervical Dystonia
2.4 Dosing in Glabellar Lines
2.5 Dosing in Spasticity in Adults
2.6 Dosing in Spasticity in Pediatric Patients
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Spread of Toxin Effect
5.2 Lack of Interchangeability between Botulinum Toxin Products
5.3 Hypersensitivity Reactions
5.4 Dysphagia and Breathing Difficulties
5.5 Facial Anatomy in the Treatment of Glabellar Lines
5.6 Dry Eye with the Treatment of Glabellar Lines
5.7 Pre-existing Neuromuscular Disorders
5.8 Human Albumin and Transmission of Viral Diseases
5.9 Intradermal Immune Reaction
5.10 Pre-existing Conditions at the Injection Site
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Aminoglycosides and Other Agents Interfering with Neuromuscular Transmission
7.2 Anticholinergic Drugs
7.3 Other Botulinum Neurotoxin Products
7.4 Muscle Relaxants
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Ethnic Groups
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Cervical Dystonia
14.2 Glabellar Lines
14.3 Spasticity in Adults
14.4 Spasticity in Pediatric Patients
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: DISTANT SPREAD OF TOXIN EFFECT
Postmarketing reports indicate that the effects of DYSPORT and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These may include asthenia, generalized muscle weakness, diplopia, blurred vision, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have underlying conditions that would predispose them to these symptoms. In unapproved uses and in approved indications, cases of spread of effect have been reported at doses comparable to or lower than the maximum recommended total dose [see Warnings and Precautions (5.1)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Instructions for Safe Use
The potency units of DYSPORT are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of DYSPORT cannot be compared to or converted into units of any other botulinum toxin products assessed with any other specific assay method [see Warnings and Precautions (5.2) and Description (11)]. Reconstituted DYSPORT is intended for intramuscular injection only.
2.2 Preparation of DYSPORT Solution for Administration
DYSPORT is supplied as a dry powder, in single-dose 300 Unit and 500 Unit vials, which must be reconstituted with preservative-free 0.9% Sodium Chloride Injection, USP using aseptic technique prior to intramuscular injection. Table 1 provides dilution instructions for the 300 Unit and 500 Unit vials, depending on the desired final concentration. The desired final concentration after dilution varies depending on the indication (see Table 2 for the recommended solution concentration after dilution).
Table 1: Dilution Instructions for DYSPORT Vials (500 Units and 300 Units) Diluent* per 500 Unit Vial Resulting Dose Units per 0.1 mL Diluent* per 300 Unit Vial Resulting Dose Units per 0.1 mL Note: These dilutions are calculated for an injection volume of 0.1 mL. A decrease or increase in the DYSPORT dose is also possible by administering a smaller or larger injection volume (i.e., 0.05 mL (50% decrease in dose), 0.08 mL (20% decrease in dose) or 0.15 mL (50% increase in dose)). - *
- Preservative-free 0.9% Sodium Chloride Injection, USP Only
- †
-
When using 5 mL of diluent for a 500 Unit vial of DYSPORT, no more than 2.5 mL of 0.9% Sodium Chloride Injection, USP should be introduced into the vial. Complete the following steps:
- Reconstitute a 500 Unit vial of DYSPORT with 2.5 mL of preservative-free 0.9% Sodium Chloride Injection, USP, gently mix, and set the vial aside.
- Withdraw 2.5 mL of preservative-free 0.9% Sodium Chloride Injection, USP, into a 5 mL syringe.
- Take the 5 mL syringe with 2.5 mL preservative-free 0.9% Sodium Chloride Injection, USP, and draw up the DYSPORT solution from the reconstituted vial without inverting and mix gently. The resulting concentration will be 10 Units/0.1 mL.
- Use immediately after reconstitution in the syringe. Dispose of any unused 0.9% Sodium Chloride Injection, USP .
1 mL 50 Units 0.6 mL 50 Units 2 mL
2.5 mL25 Units
20 Units--
1.5 mL--
20 Units-- -- 2.5 mL 12 Units 5 mL† 10 Units 3 mL 10 Units Using an appropriately sized sterile syringe, needle and aseptic technique, draw up the required amount of sterile, preservative-free 0.9% Sodium Chloride Injection, USP (see Table 1). Insert the needle into the DYSPORT vial. The partial vacuum will begin to pull the saline into the vial. Any remaining required saline should be expressed into the vial manually. Do not use the vial if no partial vacuum is observed. Swirl gently to dissolve. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
Reconstituted DYSPORT should be a clear, colorless solution, free of particulate matter, otherwise it should not be injected. Expel any air bubbles in the syringe barrel. Remove the needle used to reconstitute the product and attach an appropriately sized new sterile needle for injection.
After reconstitution, DYSPORT should be used for only one injection session and for only one patient. Discard any unused portion. Once reconstituted, unused DYSPORT may be stored in the original container, in a refrigerator at 2°C to 8°C (36°F to 46°F), protected from light for up to 24 hours until time of use. It must be discarded if not used within 24 hours. Do not freeze reconstituted DYSPORT. Discard the vial and needle in accordance with local regulations.
Table 2: Recommended Solution Concentration and Dosing Range of DYSPORT by Indication Indication Recommended Concentration Recommended DYSPORT Dose - *
- No more than 1 mL should generally be administered at any single injection site
- †
- No more than 0.5 mL of DYSPORT should be administered in any single injection site
- ‡
- Further dilution with preservative-free 0.9% Sodium Chloride Injection, USP, may be required to achieve the final volume for injection.
Cervical Dystonia, Adults 50 Units/0.1 mL
or
25 Units/0.1 mL500 Units to 1000 Units Glabellar Lines, Adults 12 Units/0.1 mL
or
20 Units/0.1 mL50 Units, divided in five equal aliquots of 10 Units (0.08 mL) each
or
50 Units, divided in five equal aliquots of 10 Units (0.05 mL) eachSpasticity, Adults* 10 Units/0.1 mL
or
20 Units/0.1 mLUpper Limb: 500 Units to 1000 Units
Lower Limb: 1000 Units to 1500 Units
Maximum total dose per treatment session = 1500 UnitsSpasticity, Pediatric Patients† 20 Units/0.1 mL
or
50 Units/0.1 mL‡Upper Limb: 8 Units/kg to 16 Units/kg per limb - Maximum total dose per treatment session = 16 Units/kg or 640 Units, whichever is lower
- Maximum total dose per treatment session for unilateral limb injections = 15 Units/kg or 1000 Units, whichever is lower
- Maximum total dose per treatment session for bilateral limb injections = 30 Units/kg or 1000 Units, whichever is lower
2.3 Dosing in Cervical Dystonia
The recommended initial dose of DYSPORT for the treatment of cervical dystonia in adults is 500 Units given intramuscularly as a divided dose among affected muscles in patients with or without a history of prior treatment with botulinum toxin. (A description of the average DYSPORT dose and percentage of total dose injected into specific muscles in the pivotal clinical trials can be found in Table 15 of Section 14.1, Clinical Studies – Cervical Dystonia.) Limiting the dose injected into the sternocleidomastoid muscle may reduce the occurrence of dysphagia. Clinical studies with DYSPORT in cervical dystonia suggest that the peak effect occurs between two and four weeks after injection. Simultaneous guided injection of DYSPORT with EMG and/or ultrasound may be helpful in locating active muscles.
Dose Modification
Where dose modification is necessary for the treatment of cervical dystonia, uncontrolled open-label studies suggest that dose adjustment can be made in 250 Unit steps according to the individual patient's response, with re-treatment every 12 weeks or longer, as necessary, based on return of clinical symptoms. Uncontrolled, open-label studies also suggest that the total dose administered in a single treatment should be between 250 Units and 1000 Units. Re-treatment, if needed, should not occur in intervals of less than 12 weeks. Doses above 1000 Units have not been systematically evaluated.
2.4 Dosing in Glabellar Lines
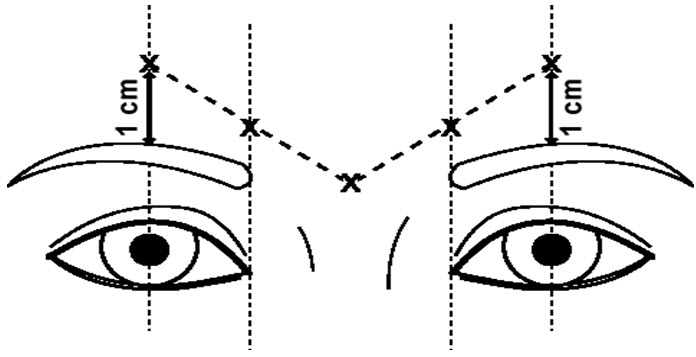
The dose of DYSPORT for the treatment of glabellar lines in adults is a total of 50 Units given intramuscularly in five equal aliquots of 10 Units each to achieve clinical effect (see Figure 1).
The clinical effect of DYSPORT may last up to four months. Repeat dosing in clinical studies demonstrated continued efficacy with up to four repeated administrations. It should be administered no more frequently than every three months. When used for re-treatment, DYSPORT should be reconstituted and injected using the same techniques as the initial treatment.
Injection Technique
Glabellar facial lines arise from the activity of the lateral corrugator and vertical procerus muscles. These can be readily identified by palpating the tensed muscle mass while having the patient frown. The corrugator depresses the skin creating a "furrowed" vertical line surrounded by tensed muscle (i.e., frown lines). The location, size, and use of the muscles vary markedly among individuals. Physicians administering DYSPORT must understand the relevant neuromuscular and/or orbital anatomy of the area involved and any alterations to the anatomy due to prior surgical procedures.
Risk of ptosis can be mitigated by careful examination of the upper lid for separation or weakness of the levator palpebrae muscle (true ptosis), identification of lash ptosis, and evaluation of the range of lid excursion while manually depressing the frontalis to assess compensation.
In order to reduce the complication of ptosis, the following steps should be taken:
- Avoid injection near the levator palpebrae superioris, particularly in patients with larger brow depressor complexes.
- Medial corrugator injections should be placed at least 1 centimeter above the bony supraorbital ridge.
- Ensure the injected volume/dose is accurate and where feasible kept to a minimum.
- Do not inject toxin closer than 1 centimeter above the central eyebrow.
To inject DYSPORT, advance the needle through the skin into the underlying muscle while applying finger pressure on the superior medial orbital rim. Inject patients with a total of 50 Units in five equally divided aliquots. Using an appropriately sized needle, inject 10 Units of DYSPORT into each of five sites, two in each corrugator muscle, and one in the procerus muscle (see Figure 1).
2.5 Dosing in Spasticity in Adults
Dosing in initial and subsequent treatment sessions should be tailored to the individual based on the size, number and location of muscles involved, severity of spasticity, the presence of local muscle weakness, the patient's response to previous treatment, and/or adverse reaction history with botulinum toxins.
No more than 1 mL should generally be administered at any single injection site. The maximum recommended total dose (upper and lower limb combined) of DYSPORT for the treatment of spasticity in adults is 1500 Units.
Although actual location of the injection sites can be determined by palpation, the use of injection guiding technique (e.g., electromyography, electrical stimulation, or ultrasound) is recommended to target the injection sites.
Upper Limb Spasticity
In the clinical trial that assessed the efficacy and safety of DYSPORT for treatment of upper limb spasticity in adults [see Clinical Studies (14.3)], doses of 500 Units and 1000 Units were divided among selected muscles at a given treatment session (see Table 3 and Figure 2).
Table 3: DYSPORT Dosing by Muscle for Upper Limb Spasticity in Adults Muscles Injected Recommended Dose DYSPORT Recommended Number of Injection(s) per Muscle Flexor carpi radialis (FCR) 100 Units to 200 Units 1 to 2 Flexor carpi ulnaris (FCU) 100 Units to 200 Units 1 to 2 Flexor digitorum profundus (FDP) 100 Units to 200 Units 1 to 2 Flexor digitorum superficialis (FDS) 100 Units to 200 Units 1 to 2 Brachialis 200 Units to 400 Units 1 to 2 Brachioradialis 100 Units to 200 Units 1 to 2 Biceps Brachii (BB) 200 Units to 400 Units 1 to 2 Pronator Teres 100 Units to 200 Units 1 Figure 2: Muscles for Injection for Upper Limb Spasticity in Adults
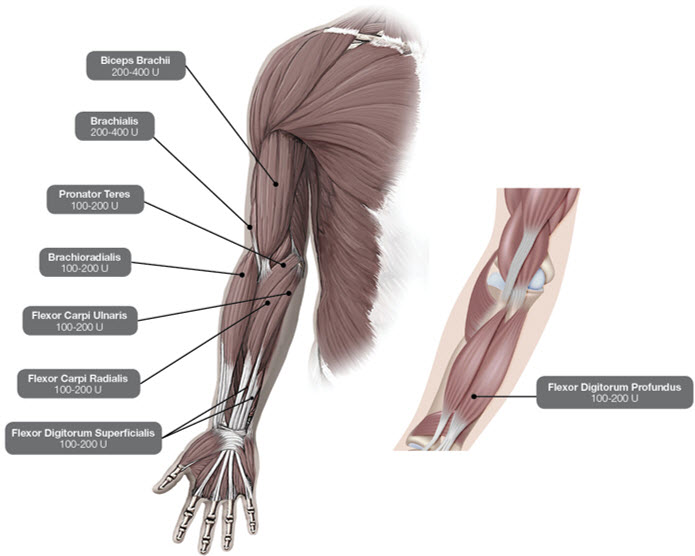
Repeat DYSPORT treatment should be administered when the effect of a previous injection has diminished, but no sooner than 12 weeks after the previous injection. A majority of patients in clinical studies were retreated between 12-16 weeks; however some patients had a longer duration of response (i.e., 20 weeks). The degree and pattern of muscle spasticity at the time of re-injection may necessitate alterations in the dose of DYSPORT and muscles to be injected. Clinical improvement may be expected one week after administration of DYSPORT.
Lower Limb Spasticity
In the clinical trial that assessed the efficacy and safety of DYSPORT for treatment of lower limb spasticity in adults [see Clinical Studies (14.3)], doses of 1000 Units and 1500 Units were divided among selected muscles at a given treatment session (see Table 4 and Figure 3).
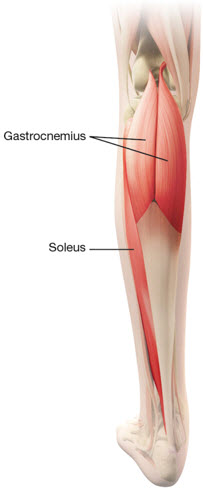
Table 4: DYSPORT Dosing by Muscle for Lower Limb Spasticity in Adults Muscles Injected Recommended DYSPORT Dose Recommended Number of Injection Sites per Muscle Distal Muscles Gastrocnemius Medial head 100 Units to 150 Units 1 Lateral head 100 Units to 150 Units 1 Soleus 330 Units to 500 Units 3 Tibialis posterior 200 Units to 300 Units 2 Flexor digitorum longus 130 Units to 200 Units 1 to 2 Flexor hallucis longus 70 Units to 200 Units 1 Figure 3: Muscles for Injection for Lower Limb Spasticity in Adults
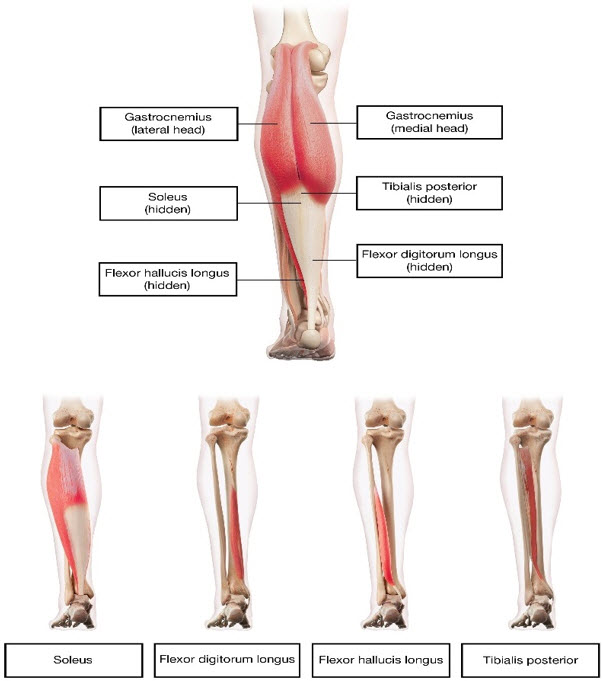
Repeat DYSPORT treatment should be administered when the effect of a previous injection has diminished, but no sooner than 12 weeks after the previous injection. A majority of patients in clinical studies were retreated between 12-16 weeks. The degree and pattern of muscle spasticity at the time of re-injection may necessitate alterations in the dose of DYSPORT and muscles to be injected.
2.6 Dosing in Spasticity in Pediatric Patients
DYSPORT dosing for spasticity in pediatric patients is based on Units per kilogram of body weight. To calculate the total units of DYSPORT required for treatment of one limb, select the dose of DYSPORT in Units/kg and the body weight (kg) of the patient (see Tables 5 and 6). Dosing in initial and sequential treatment sessions should be tailored to the individual patient based on the size, number and location of muscles involved, severity of spasticity, the presence of local muscle weakness, the patient's response to previous treatment, and/or adverse reaction history with botulinum toxins.
No more than 0.5 mL should generally be administered at any single injection site. The maximum recommended total dose of DYSPORT in a single treatment session for spasticity in pediatric patients 2 years and older is 30 Units/kg or 1000 Units in a 3-month interval.
Although actual location of the injection sites can be determined by palpation, the use of injection guiding technique (e.g., electromyography or electrical stimulation, or ultrasound) is recommended to target the injection sites.
Upper Limb Spasticity in Pediatric Patients 2 Years of Age and Older
In the clinical trial that assessed the efficacy and safety of DYSPORT for treatment of upper limb spasticity in pediatric patients 2 years of age or older with a weight of at least 10 kg [see Clinical Studies (14.4)], doses of 8 Units/kg or 16 Units/kg were divided among selected muscles of the target upper limb at a given treatment session (see Table 5 and Figure 4).
Table 5 describes the recommended Units/kg dose of DYSPORT per muscle. The maximum recommended total dose of DYSPORT administered for treatment of upper limb spasticity must not exceed 16 Units/kg or 640 Units, whichever is lower.
Table 5: DYSPORT Dosing by Muscle for Upper Limb Spasticity in Pediatric Patients Muscle Recommended Dose Range
per muscle per upper limb
(Units/kg Body Weight)Number of injection sites
per muscleBrachialis 3 Units/kg to 6 Units/kg Up to 2 Brachioradialis 1.5 Units/kg to 3 Units/kg 1 Biceps brachii 3 Units/kg to 6 Units/kg Up to 2 Pronator teres 1 Units/kg to 2 Units/kg 1 Pronator quadratus 0.5 Units/kg to 1 Units/kg 1 Flexor carpi radialis (FCR) 2 Units/kg to 4 Units/kg Up to 2 Flexor carpi ulnaris (FCU) 1.5 Units/kg to 3 Units/kg 1 Flexor digitorum profundus (FDP) 1 Units/kg to 2 Units/kg 1 Flexor digitorum superficialis (FDS) 1.5 Units/kg to 3 Units/kg Up to 4 Total dose 8 Units/kg to 16 Units/kg in upper limbs (and not exceeding 640 Units) Figure 4: Muscles for Injection for Upper Limb Spasticity in Pediatric Patients
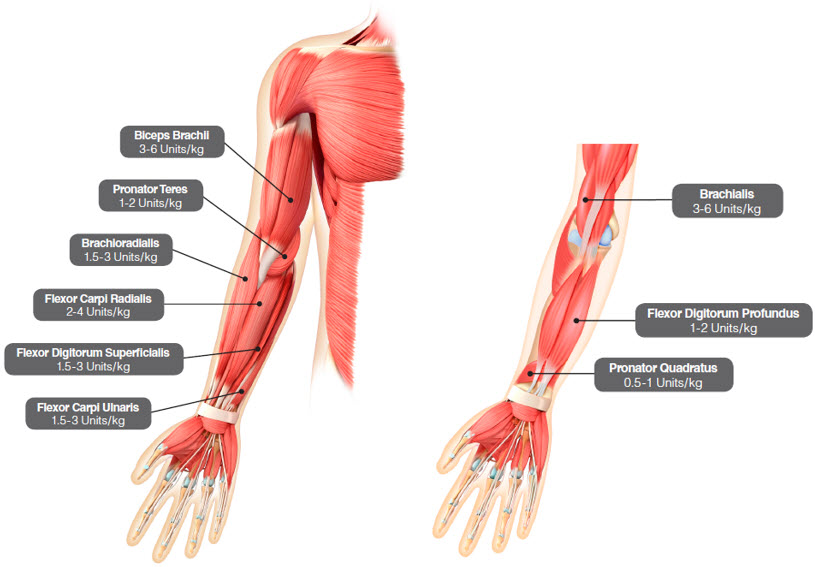
Repeat DYSPORT treatment should be administered when the effect of a previous injection has diminished but no sooner than 16 weeks after the previous injection. A majority of patients in the clinical study were retreated between 16-28 weeks; however, some patients had a longer duration of response (i.e., 34 weeks or more). The degree and pattern of muscle spasticity at the time of re-injection may necessitate alterations in the dose of DYSPORT and muscles to be injected.
Lower Limb Spasticity in Pediatric Patients 2 Years of Age and Older In the clinical trial that assessed the efficacy and safety of DYSPORT for treatment of lower limb spasticity in pediatric patients 2 years of age or older [see Clinical Studies (14.4)], doses of 10 Units/kg to 15 Units/kg were divided among selected muscles of the target lower limb at a given treatment session (see Table 6 and Figure 5).
Table 6 describes the recommended Units/kg dose of DYSPORT per muscle of the Gastrocnemius-Soleus Complex (GSC). The recommended total DYSPORT dose per treatment session is 10 Units/kg to 15 Units/kg for unilateral lower limb injections or 20 Units/kg to 30 Units/kg for bilateral lower limb injections. However, the total dose of DYSPORT administered in a 3-month interval must not exceed 15 Units/kg for unilateral lower limb injections, 30 Units/kg for bilateral lower limb injections, or 1000 units, whichever is lower. The total dose administered should be divided between the affected spastic muscles of the lower limb(s). When possible, the dose should be distributed across more than 1 injection site in any single muscle (see Table 6).
Table 6: DYSPORT Dosing by Muscle for Lower Limb Spasticity in Pediatric Patients Muscle Injected Recommended DYSPORT Dose Range per muscle per leg (Units/kg Body Weight) Recommended number of injections per muscle - *
- the listed individual doses to be injected in the muscles can be used within the range mentioned without exceeding 15 Units/kg total dose for unilateral injection or 30 Units/kg for bilateral injections or 1000 Units whichever is lower.
Gastrocnemius 6 Units/kg to 9 Units/kg* Up to 4 Soleus 4 Units/kg to 6 Units/kg* Up to 2 Total 10 Units/kg to 15 Units/kg divided across both muscles Up to 6 Figure 5: Muscles for Injection for Lower Limb Spasticity in Pediatric Patients
Although actual location of the injection sites can be determined by palpation, the use of injection guiding technique (e.g., electromyography or electrical stimulation, or ultrasound) is recommended to target the injection sites.
Repeat DYSPORT treatment should be administered when the effect of a previous injection has diminished but no sooner than 12 weeks after the previous injection. A majority of patients in the clinical studies were retreated between 16-22 weeks, however; some had a longer duration of response. The degree and pattern of muscle spasticity and overall clinical benefit at the time of re-injection may necessitate alterations in the dose of DYSPORT and muscles to be injected.
The safety and effectiveness of DYSPORT injected into proximal muscles of the lower limb for the treatment of spasticity in pediatric patients has not been established.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
DYSPORT is contraindicated in patients with:
- Known hypersensitivity to any botulinum toxin products, cow's milk protein, or to any of the components in the formulation [see Warnings and Precautions (5.3)]. This product may contain trace amounts of cow's milk protein [see Description (11)].
- Infection at the proposed injection site(s).
-
5 WARNINGS AND PRECAUTIONS
5.1 Spread of Toxin Effect
Postmarketing safety data from DYSPORT and other approved botulinum toxins suggest that botulinum toxin effects may, in some cases, be observed beyond the site of local injection. The symptoms are consistent with the mechanism of action of botulinum toxin and may include asthenia, generalized muscle weakness, diplopia, blurred vision, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life-threatening and there have been reports of death related to spread of toxin effects. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have underlying conditions that would predispose them to these symptoms. In unapproved uses and approved indications, symptoms consistent with spread of toxin effect have been reported at doses comparable to or lower than the maximum recommended total dose .
5.2 Lack of Interchangeability between Botulinum Toxin Products
The potency Units of DYSPORT are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of DYSPORT cannot be compared to or converted into units of any other botulinum toxin products assessed with any other specific assay method [see Description (11)].
5.3 Hypersensitivity Reactions
Serious hypersensitivity reactions have been reported with DYSPORT. Hypersensitivity reactions include anaphylaxis, serum sickness, urticaria, soft tissue edema, and dyspnea. If such a serious hypersensitivity reaction occurs, discontinue further injection of DYSPORT and institute appropriate medical therapy immediately.
5.4 Dysphagia and Breathing Difficulties
Treatment with DYSPORT and other botulinum toxin products can result in swallowing or breathing difficulties. Patients with pre-existing swallowing or breathing difficulties may be more susceptible to these complications. In most cases, this is a consequence of weakening of muscles in the area of injection that are involved in breathing or swallowing. When distant effects occur, additional respiratory muscles may be involved [see Boxed Warning and Warnings and Precautions (5.2)].
Deaths as a complication of severe dysphagia have been reported after treatment with botulinum toxin. Dysphagia may persist for several weeks and require use of a feeding tube to maintain adequate nutrition and hydration. Aspiration may result from severe dysphagia and is a particular risk when treating patients in whom swallowing or respiratory function is already compromised.
Treatment of cervical dystonia with botulinum toxins may weaken neck muscles that serve as accessory muscles of ventilation. This may result in a critical loss of breathing capacity in patients with respiratory disorders who may have become dependent upon these accessory muscles. There have been post-marketing reports of serious breathing difficulties, including respiratory failure.
Patients treated with botulinum toxin may require immediate medical attention should they develop problems with swallowing, speech or respiratory disorders. These reactions can occur within hours to weeks after injection with botulinum toxin [see Boxed Warning, Warnings and Precautions (5.2), Adverse Reactions (6.1), Clinical Pharmacology (12.2)].
5.5 Facial Anatomy in the Treatment of Glabellar Lines
Caution should be exercised when administering DYSPORT to patients with surgical alterations to the facial anatomy, marked facial asymmetry, inflammation at the injection site(s), ptosis, excessive dermatochalasis, deep dermal scarring, thick sebaceous skin [see Dosage and Administration (2.4)] or the inability to substantially lessen glabellar lines by physically spreading them apart [see Clinical Studies (14.2)].
Do not exceed the recommended dosage and frequency of administration of DYSPORT. In clinical trials, subjects who received a higher dose of DYSPORT had an increased incidence of eyelid ptosis.
5.6 Dry Eye with the Treatment of Glabellar Lines
Dry eye has been reported with the use of DYSPORT in the treatment of glabellar lines [see Adverse Reactions (6.3)]. Reduced tear production, reduced blinking, and corneal disorders, may occur with use of botulinum toxins, including DYSPORT. If symptoms of dry eye (e.g., eye irritation, photophobia, or visual changes) persist, consider referring patient to an ophthalmologist [see Boxed Warning and Warnings and Precautions 5.2].
5.7 Pre-existing Neuromuscular Disorders
Individuals with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis or neuromuscular junction disorders (e.g., myasthenia gravis or Lambert-Eaton syndrome) should be monitored particularly closely when given botulinum toxin. Patients with neuromuscular disorders may be at increased risk of clinically significant effects including severe dysphagia and respiratory compromise from typical doses of DYSPORT [see Adverse Reactions (6.1)].
5.8 Human Albumin and Transmission of Viral Diseases
This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases and variant Creutzfeldt-Jakob disease (vCJD). There is a theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD), but if that risk actually exists, the risk of transmission would also be considered extremely remote. No cases of transmission of viral diseases, CJD, or vCJD have ever been identified for licensed albumin or albumin contained in other licensed products.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed below and elsewhere in labeling:
- Spread of Toxin Effect [see Warnings and Precautions (5.1)]
- Lack of Interchangeability between Botulinum Toxin Products [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Dysphagia and Breathing Difficulties [see Warnings and Precautions (5.4)]
- Facial Anatomy in the Treatment of Glabellar Lines [see Warnings and Precautions (5.5)]
- Dry Eye with the Treatment of Glabellar Lines [see Warnings and Precautions (5.6)]
- Pre-existing Neuromuscular Disorders [see Warnings and Precautions (5.7)]
- Human Albumin and Transmission of Viral Diseases [see Warnings and Precautions (5.8)]
- Intradermal Immune Reaction [see Warnings and Precautions (5.9)]
- Pre-existing Conditions at the Injection Site [see Warnings and Precautions (5.10)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Cervical Dystonia
The data described below reflect exposure to DYSPORT in 446 cervical dystonia patients in 7 studies. Of these, two studies were randomized, double-blind, single treatment, placebo-controlled studies with subsequent optional open-label treatment in which dose optimization (250 to 1000 Units per treatment) over the course of 5 treatment cycles was allowed [see Clinical Studies (14.1)].
The population was almost entirely Caucasian (99%) with a median age of 51 years (range 18–82 years). Most patients (87%) were less than 65 years of age; 58.4% were women.
Common Adverse Reactions
The most commonly reported adverse reactions (occurring in 5% or more of patients who received 500 Units of DYSPORT in the placebo-controlled clinical trials) in cervical dystonia patients were: muscular weakness, dysphagia, dry mouth, injection site discomfort, fatigue, headache, musculoskeletal pain, dysphonia, injection site pain and eye disorders (consisting of blurred vision, diplopia, and reduced visual acuity and accommodation). Other than injection site reactions, most adverse reactions became noticeable about one week after treatment and lasted several weeks.
The rates of adverse reactions were higher in the combined controlled and open-label experience than in the placebo-controlled trials.
During the clinical studies, two patients (<1%) experienced adverse reactions leading to withdrawal. One patient experienced disturbance in attention, eyelid disorder, feeling abnormal and headache, and one patient experienced dysphagia.
Table 7 compares the incidence of the most frequent adverse reactions from a single treatment cycle of 500 Units of DYSPORT compared to placebo [see Clinical Studies (14.1)].
Table 7: Most Common Adverse Reactions (≥5%) and Greater than Placebo in the Pooled, Double-Blind, Placebo-Controlled Phase of Clinical Trials in Patients with Cervical Dystonia Adverse Reactions DYSPORT 500 Units
(N=173)
%Placebo
(N=182)
%Any Adverse Reaction 61 51 - *
- The following preferred terms were reported: vision blurred, diplopia, visual acuity reduced, eye pain, eyelid disorder, accommodation disorder, dry eye, eye pruritus.
General disorders and administration site conditions Injection site discomfort 13 8 Fatigue 12 10 Injection site pain 5 4 Musculoskeletal and connective tissue disorders Muscular weakness 16 4 Musculoskeletal pain 7 3 Gastrointestinal disorders Dysphagia 15 4 Dry mouth 13 7 Nervous system disorders Headache 11 9 Respiratory, thoracic and mediastinal disorders Dysphonia 6 2 Eye Disorders* 7 2 Dose-response relationships for common adverse reactions in a randomized multiple fixed-dose study in which the total dose was divided between two muscles (the sternocleidomastoid and splenius capitis) are shown in Table 8.
Table 8: Common Adverse Reactions by Dose in Fixed-Dose Study in Patients with Cervical Dystonia
Adverse ReactionsDYSPORT Dose 250 Units
%500 Units
%1000 Units
%Placebo
%- *
- The following preferred terms were reported: vision blurred, diplopia, visual acuity reduced, eye pain, eyelid disorder, accommodation disorder, dry eye, eye pruritus.
Any Adverse Reaction 37 65 83 30 Dysphagia 21 29 39 5 Dry Mouth 21 18 39 10 Muscular Weakness 11 12 56 0 Injection Site Discomfort 5 18 22 10 Dysphonia 0 18 28 0 Facial Paresis 5 0 11 0 Eye Disorders* 0 6 17 0 Less Common Adverse Reactions
The following adverse reactions were reported less frequently (<5%).
Breathing Difficulty
Breathing difficulties were reported by approximately 3% of patients following DYSPORT administration and in 1% of placebo patients in clinical trials during the double-blind phase. These consisted mainly of dyspnea. The median time to onset from last dose of DYSPORT was approximately one week, and the median duration was approximately three weeks.
Other adverse reactions with incidences of less than 5% in the DYSPORT 500 Units group in the double-blind phase of clinical trials included dizziness in 3.5% of DYSPORT-treated patients and 1% of placebo-treated patients, and muscle atrophy in 1% of DYSPORT-treated patients and in none of the placebo-treated patients.
Laboratory Findings
Patients treated with DYSPORT exhibited a small increase from baseline (0.23 mol/L) in mean blood glucose relative to placebo-treated patients. This was not clinically significant among patients in the development program but could be a factor in patients whose diabetes is difficult to control.
Electrocardiographic Findings
ECG measurements were only recorded in a limited number of patients in an open-label study without a placebo or active control. This study showed a statistically significant reduction in heart rate compared to baseline, averaging about three beats per minute, observed thirty minutes after injection.
Glabellar Lines
In placebo-controlled clinical trials of DYSPORT, the most common adverse reactions (≥2%) following injection of DYSPORT were nasopharyngitis, headache, injection site pain, injection site reaction, upper respiratory tract infection, eyelid edema, eyelid ptosis, sinusitis, nausea, and blood present in urine.
Table 9 reflects exposure to DYSPORT in 398 patients 19 to 75 years of age who were evaluated in the randomized, placebo-controlled clinical studies that assessed the use of DYSPORT for the temporary improvement in the appearance of glabellar lines [see Clinical Studies (14.2)]. Adverse reactions of any cause occurred in 48% of the DYSPORT-treated patients and 33% of the placebo-treated patients.
Table 9: Most Common Adverse Reactions with > 1% Incidence in Pooled, Placebo-Controlled Trials for Glabellar Lines Adverse Reactions by Body System DYSPORT
(N=398)
%*Placebo
(N=496)
%*- *
- Patients who received treatment with placebo and DYSPORT are counted in both treatment columns.
Any Adverse Reaction 48 33 Eye Disorders Eyelid Edema 2 0 Eyelid Ptosis 2 <1 Gastrointestinal Disorders Nausea 2 1 General Disorders and Administration Site Conditions Injection Site Pain 3 2 Injection Site Reaction 3 <1 Infections and Infestations Nasopharyngitis 10 4 Upper Respiratory Tract Infection 3 2 Sinusitis 2 1 Investigations Blood Present in Urine 2 <1 Nervous System Disorders Headache 9 5 In the clinical trials safety database, where some patients received up to twelve treatments with DYSPORT, adverse reactions were reported for 57% (1425/2491) of patients. The most frequently reported of these adverse reactions were headache, nasopharyngitis, injection site pain, sinusitis, URI, injection site bruising, and injection site reaction (numbness, discomfort, erythema, tenderness, tingling, itching, stinging, warmth, irritation, tightness, swelling).
Adverse reactions that occurred after repeated injections in 2–3% of the population included bronchitis, influenza, pharyngolaryngeal pain, cough, contact dermatitis, injection site swelling, and injection site discomfort.
The incidence of eyelid ptosis did not increase in the long-term safety studies with multiple re-treatments at intervals ≥ three months. The majority of the reports of eyelid ptosis were mild to moderate in severity and resolved over several weeks [see Dosage and Administration (2.4)].
Spasticity in Adults
Injection Site Reactions
Injection site reactions (e.g., pain, bruising, hemorrhage, erythema/hematoma, etc.) have occurred following administration of DYSPORT in adults treated for spasticity.
Upper Limb Spasticity in Adults
Table 10 lists the adverse reactions that occurred in ≥2% of patients in any DYSPORT dose group and more frequent than placebo in double-blind studies evaluating the treatment of upper limb spasticity in adults. The most common adverse reactions (≥4%) in any DYSPORT dose group was muscular weakness.
Table 10: Most Common Adverse Reactions Observed in at Least 2% of Patients Treated in Pooled, Double-Blind Trials of Adults with Upper Limb Spasticity Reported More Frequently than with Placebo DYSPORT Placebo Adverse Reactions 500 Units
(N=197)
%1000 Units
(N=194)
%
(N=279)
%Infections and infestations Influenza 1 2 1 Infection 1 2 1 Musculoskeletal and connective tissue disorders Muscular weakness 2 4 1 Pain in extremity 0 2 1 Back pain 1 2 1 Nervous system disorders Headache 1 2 1 Convulsion 2 2 1 Syncope 1 2 0 Hypoesthesia 0 2 <1 Partial seizures 0 2 0 General disorders and administration site conditions Fatigue 2 2 0 Asthenia 2 1 <1 Injury, poisoning and procedural complications Fall 2 3 2 Injury 2 2 1 Contusion 1 2 <1 Gastrointestinal disorders Diarrhea 1 2 <1 Constipation 0 2 1 Investigation Blood triglycerides increased 2 1 0 Respiratory, thoracic and mediastinal disorders Cough 1 2 1 Vascular disorders Hypertension 1 2 <1 Psychiatric disorders Depression 2 3 1 Lower Limb Spasticity in Adults
The data described below reflect exposure to DYSPORT in 255 adults with lower limb spasticity. Of this population, 89% were Caucasian, 66% male, and the median age was 55 years (range 23–77 years). Table 11 lists the adverse reactions that occurred in ≥2% of patients in any DYSPORT dose group and more frequent than placebo in the double-blind study evaluating the treatment of lower limb spasticity in adults. The most common of these adverse reactions (≥5%) in any DYSPORT dose group were falls, muscular weakness, and pain in extremity.
Table 11: Adverse Reactions Observed in at Least 2% of Patients Treated in the Double-Blind Trial of Adults with Lower Limb Spasticity and Reported More Frequently than with Placebo Adverse Reactions DYSPORT 1000 U
(N=127)
%DYSPORT 1500 U (N=128)
%Placebo
(N=130)
%Musculoskeletal and connective tissue disorders Muscular weakness 2 7 3 Pain in extremity 6 6 2 Arthralgia 4 2 1 Injury, poisoning and procedural complications Fall 9 6 3 Nervous system disorders Headache 0 3 1 General disorders and administration site conditions Fatigue 1 4 0 Influenza-like illness 2 0 0 Edema peripheral 2 0 0 Investigations Alanine aminotransferase increased 2 0 1 Gastrointestinal disorders Constipation 0 2 1 Psychiatric disorders Depression 2 3 0 Insomnia 0 2 0 In the efficacy and safety studies of DYSPORT for the treatment of lower limb spasticity in adults, muscular weakness was reported more frequently in women (10%) treated with 1500 units of DYSPORT compared to men (5%). Falls were reported more frequently in patients 65 years of age and over [see Use in Specific Populations (8.5)].
Upper Limb Spasticity in Pediatric Patients
Table 12 reflects exposure to DYSPORT in 210 patients, 2 to 17 years of age, who were evaluated in a double blind, active-controlled, multicenter study in patients treated for upper limb spasticity [see Clinical Studies (14.4)]. The most commonly observed adverse reactions (≥10% of patients) were: upper respiratory tract infection and pharyngitis.
Table 12: Adverse Reactions Observed in ≥ 3% of Patients Treated in Double-Blind Study of Pediatric Patients with Upper Limb Spasticity and Reported More Frequently than Control Group Adverse Reactions DYSPORT 2 Units/kg*
(N=70)
%DYSPORT 8 Units/kg
(N=70)
%DYSPORT 16 Units/kg
(N=70)
%Infections and infestations Upper respiratory tract infection 7 9 11 Influenza 1 1 3 Pharyngitis† 9 6 10 Gastrointestinal disorders Nausea 0 3 1 Musculoskeletal and connective tissue disorders Muscular weakness 1 4 6 Nervous system disorders Headache 0 6 3 Epilepsy 1 0 4 Additional adverse reactions occurring below 3% and considered to be drug related include: myalgia, pain in extremity, fatigue, influenza-like illness, injection site eczema, injection site bruising, injection site rash, injection site pain, and injection site swelling.
Lower Limb Spasticity in Pediatric Patients
Table 13 reflects exposure to DYSPORT in 160 patients, 2 to 17 years of age, who were evaluated in the randomized, placebo-controlled clinical study that assessed the use of DYSPORT for the treatment of unilateral or bilateral lower limb spasticity in pediatric cerebral palsy patients [see Clinical Studies (14.4)]. The most commonly observed adverse reactions (≥10% of patients) were: upper respiratory tract infection, nasopharyngitis, influenza, pharyngitis, cough and pyrexia.
Table 13: Adverse Reactions Observed in ≥ 4% of Patients Treated in the Double-Blind Trial of Pediatric Patients with Lower Limb Spasticity and Reported More Frequently than with Placebo Adverse Reactions Unilateral Bilateral DYSPORT
10 Units/kg
(N=43)
%DYSPORT
15 Units/kg
(N=50)
%DYSPORT
20 Units/kg
(N=37)
%DYSPORT
30 Units/kg
(N=30)
%Placebo
(N=79)
%Infections and infestations Nasopharyngitis 9 12 16 10 5 Bronchitis 0 0 8 7 3 Respiratory, thoracic and mediastinal disorders Cough 7 6 14 10 6 General disorders and administration site conditions Pyrexia 7 12 8 7 5 Musculoskeletal and connective tissue disorders Pain in extremity 0 2 5 7 5 Nervous system disorders Convulsion/Epilepsy 7 4 0 7 0 6.2 Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity.
The incidence of antibody formation is highly dependent on the sensitivity and specificity of the assay. In addition, the observed incidence of antibody positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies across products in this class may be misleading.
Cervical Dystonia
About 3% of subjects developed antibodies (binding or neutralizing) over time with DYSPORT treatment.
Glabellar Lines
Testing for antibodies to DYSPORT was performed for 1554 subjects who had up to nine cycles of treatment. Two subjects (0.13%) tested positive for binding antibodies at baseline. Three additional subjects tested positive for binding antibodies after receiving DYSPORT treatment. None of the subjects tested positive for neutralizing antibodies.
Spasticity in Adults
Upper Limb Spasticity
From 230 subjects treated with DYSPORT and tested for the presence of binding antibodies, 5 subjects were positive at baseline and 17 developed antibodies after treatment. Among those 17 subjects, 10 subjects developed neutralizing antibodies. An additional 51 subjects from a separate repeat-dose study were tested for the presence of neutralizing antibodies only. None of the subjects tested positive.
In total, from the 281 subjects treated in the long-term studies and tested for the presence of neutralizing antibodies, 3.6% developed neutralizing antibodies after treatment. In the presence of binding and neutralizing antibodies to DYSPORT some patients continued to experience clinical benefit.
Lower Limb Spasticity
From 367 subjects treated with DYSPORT and tested for the presence of binding antibodies, 4 subjects were positive at baseline and 2 developed binding antibodies after treatment. No subjects developed neutralizing antibodies. An additional 85 subjects from two separate studies were tested for the presence of neutralizing antibodies only. One subject tested positive for the presence of neutralizing antibodies.
In total, from the 452 subjects treated with DYSPORT and tested for the presence of neutralizing antibodies, 0.2% developed neutralizing antibodies after treatment.
Spasticity in Pediatric Patients 2 Years of Age and Older
Upper Limb Spasticity
From 178 subjects treated with DYSPORT for up to 4 treatment cycles and tested for the presence of binding antibodies at baseline and end of study, 7 subjects previously receiving botulinum toxin injections had binding antibodies after treatment. Among those 7 subjects, 4 subjects (2.3%) developed neutralizing antibodies when tested in the mice bioassay. In the presence of binding and/or neutralizing antibodies to DYSPORT some patients continue to experience clinical benefit.
Lower Limb Spasticity
From 226 subjects treated with DYSPORT and tested for the presence of binding antibodies, 5 subjects previously receiving botulinum toxins were positive at baseline and 9 patients developed binding antibodies after injections. Among those 9 subjects, 3 subjects developed neutralizing antibodies, while one subject developed neutralizing antibodies from the 5 subjects testing positive for binding antibodies at baseline who previously received botulinum toxin injections.
From a separate repeat-dose study, 203 subjects were tested for the presence of neutralizing antibodies. Two subjects were positive for neutralizing antibodies at baseline and 5 subjects developed neutralizing antibodies after treatments. In total, from the 429 patients tested for the presence of neutralizing antibodies, 2.1% developed neutralizing antibodies after treatment. In the presence of binding and neutralizing antibodies to DYSPORT, some patients continued to experience clinical benefit.
6.3 Postmarketing Experience
Because adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following adverse reactions have been identified during post-approval use of DYSPORT: vertigo, photophobia, influenza-like illness, amyotrophy, muscle atrophy, burning sensation, facial paresis, hypoesthesia, erythema, dry eye, and excessive granulation tissue. Hypersensitivity reactions including anaphylaxis have been reported.
-
7 DRUG INTERACTIONS
7.1 Aminoglycosides and Other Agents Interfering with Neuromuscular Transmission
Co-administration of DYSPORT and aminoglycosides or other agents interfering with neuromuscular transmission (e.g., curare-like agents) should only be performed with caution because the effect of the botulinum toxin may be potentiated. If co-administered, observe the patient closely.
7.2 Anticholinergic Drugs
Use of anticholinergic drugs after administration of DYSPORT may potentiate systemic anticholinergic effects such as blurred vision.
7.3 Other Botulinum Neurotoxin Products
The effect of administering botulinum neurotoxin products including DYSPORT, at the same time or within several months of each other is unknown. Excessive weakness may be exacerbated by another administration of botulinum toxin prior to the resolution of the effects of a previously administered botulinum toxin.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled clinical studies with DYSPORT in pregnant women.
DYSPORT should only be used during pregnancy if the potential benefit justifies the potential risk to the fetus.
DYSPORT produced embryo-fetal toxicity in relation to maternal toxicity when given to pregnant rats and rabbits at doses lower than or similar to the maximum recommended human dose (MRHD) of 1000 Units on a body weight (Units/kg) basis (see Data).
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively. The background risk of major birth defects and miscarriage for the indicated populations is unknown.
Data
Animal Data
In a study in which pregnant rats received daily intramuscular injections of DYSPORT (2.2, 6.6, or 22 Units/kg on gestation days 6 through 17 or intermittently 44 Units/kg on gestation days 6 and 12 only) during organogenesis, increased early embryonic death was observed with both schedules at the highest tested doses (22 and 44 Units/kg), which were associated with maternal toxicity. The no-effect dose for embryo-fetal developmental toxicity was 2.2 Units/kg (less than the maximum recommended human dose [MRHD] on a body weight basis).
In a study in which pregnant rabbits received daily intramuscular injections of DYSPORT (0.3, 3.3, or 6.7 Units/kg) on gestation days 6 through 19 or intermittently (13.3 Units/kg on gestation days 6 and 13 only) during organogenesis, no embryofetal data were available at the highest dose administered daily (6.7 Units/kg) because of premature death in all doses at that dose. At the lower daily doses or with intermittent dosing, no adverse developmental effects were observed. All doses for which data were available are less than the MRHD on a body weight basis.
In a study in which pregnant rats received 6 weekly intramuscular injections of DYSPORT (4.4, 11.1, 22.2, or 44 Units/kg) beginning on day 6 of gestation and continuing through parturition to weaning, an increase in stillbirths was observed at the highest dose tested, which was maternally toxic. The no-effect dose for pre- and post-natal developmental toxicity was 22.2 Units/kg (similar to the MRHD).
8.2 Lactation
Risk Summary
There are no data on the presence of DYSPORT in human or animal milk, the effects on the breastfed infant, or the effects on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for DYSPORT and any potential adverse effects on the breastfed infant from DYSPORT or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Infertility
In rats, DYSPORT produced adverse effects on mating behavior and fertility [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Cervical Dystonia
Safety and effectiveness in pediatric patients have not been established [see Boxed Warning and Warnings and Precautions (5.2)].
Spasticity
Safety and effectiveness have been established in pediatric patients 2 years of age and older [see Warnings and Precautions (5.1), Adverse Reactions (6.1), and Clinical Studies (14.4)]. The safety and effectiveness of DYSPORT have been established by evidence from adequate and well-controlled studies of DYSPORT in patients 2 years of age and older with upper and lower limb spasticity. The safety and effectiveness of DYSPORT injected into proximal muscles of the lower limb for the treatment of spasticity in pediatric patients has not been established [see Boxed Warning, Warnings and Precautions (5.2) and Adverse Reactions (6.1)].
Safety and effectiveness in pediatric patients below the age of 2 years have not been established [see Boxed Warning and Warnings and Precautions (5.2)].
Juvenile Animal Data
In a study in which juvenile rats received a single intramuscular injection of DYSPORT (1, 3, or 10 Units/animal) on postnatal day 21, decreased growth and bone length (injected and contralateral limbs), delayed sexual maturation, and decreased fertility were observed at the highest dose tested, which was associated with excessive toxicity during the first week after dosing.
In a study in which juvenile rats received weekly intramuscular injections of DYSPORT (0.1, 0.3, or 1.0 Units/animal) from postnatal day 21 to 13 weeks of age, decreases in bone mineral content in the injected limb, associated with atrophy of injected and adjacent muscles, were observed at the highest dose tested. No adverse effects were observed on neurobehavioral development. However, dose levels were not adjusted for growth of the pups. On a body weight basis, the doses at the end of the dosing period were approximately 15% of those at initiation of dosing. Therefore, the effects of DYSPORT throughout postnatal development were not adequately evaluated.
8.5 Geriatric Use
Cervical Dystonia
There were insufficient numbers of patients aged 65 years and over in the clinical studies to determine whether they respond differently than younger patients. In general, elderly patients should be observed to evaluate their tolerability of DYSPORT, due to the greater frequency of concomitant disease and other drug therapy [see Dosage and Administration (2.3)].
Glabellar Lines
Of the total number of subjects in the placebo-controlled clinical studies of DYSPORT, 8 (1%) were 65 years and over. Efficacy was not observed in subjects aged 65 years and over [see Clinical Studies (14.2)]. For the entire safety database of geriatric subjects, although there was no increase in the incidence of eyelid ptosis, geriatric subjects did have an increase in the number of ocular adverse reactions compared to younger subjects (11% vs. 5%) [see Dosage and Administration (2.4)].
Adult Spasticity
Upper Limb Spasticity
Of the total number of subjects in placebo-controlled clinical studies of DYSPORT, 30 percent were aged 65 years and over, while 8 percent were aged 75 years and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Lower Limb Spasticity
Of the total number of subjects in placebo controlled clinical studies of DYSPORT, 18% (n = 115) were 65 and over, while 3% (n = 20) were 75 and over. Subjects aged 65 years and over who were treated with DYSPORT reported a greater percentage of adverse reactions as compared to younger subjects (46% versus 39%). Fall and asthenia were observed with greater frequency in older subjects, as compared to those younger (10% versus 6% and 4% versus 2%, respectively).
-
10 OVERDOSAGE
Excessive doses of DYSPORT may be expected to produce neuromuscular weakness with a variety of symptoms. Respiratory support may be required where excessive doses cause paralysis of respiratory muscles. In the event of overdose, the patient should be medically monitored for symptoms of excessive muscle weakness or muscle paralysis [see Boxed Warning and Warnings and Precautions (5.2)]. Symptomatic treatment may be necessary.
Symptoms of overdose are likely not to be present immediately following injection. Should accidental injection or oral ingestion occur, the person should be medically supervised for several weeks for signs and symptoms of excessive muscle weakness or paralysis.
There is no significant information regarding overdose from clinical studies.
In the event of overdose, antitoxin raised against botulinum toxin is available from the Centers for Disease Control and Prevention (CDC) in Atlanta, GA. However, the antitoxin will not reverse any botulinum toxin-induced effects already apparent by the time of antitoxin administration. In the event of suspected or actual cases of botulinum toxin poisoning, please contact your local or state Health Department to process a request for antitoxin through the CDC. If you do not receive a response within 30 minutes, please contact the CDC directly at 770-488-7100. More information can be obtained at https://www.cdc.gov/laboratory/drugservice/index.html.
-
11 DESCRIPTION
Botulinum toxin type A, the active ingredient in DYSPORT, is a purified neurotoxin type A complex produced by fermentation of the bacterium Clostridium botulinum type A, Hall Strain. It is purified from the culture supernatant by a series of precipitation, dialysis, and chromatography steps. The neurotoxin complex is composed of the neurotoxin, hemagglutinin proteins and non-toxin non-hemagglutinin protein.
DYSPORT® (abobotulinumtoxinA) for injection is a sterile, lyophilized powder supplied in a single-dose vial for reconstitution intended for intramuscular injection. Each vial contains 300 Units or 500 Units of lyophilized abobotulinumtoxinA, human serum albumin (125 mcg) and lactose (2.5 mg). DYSPORT may contain trace amounts of cow's milk proteins [see Contraindications (4) and Warnings and Precautions (5.3)].
The primary release procedure for DYSPORT uses a cell-based potency assay to determine the potency relative to a reference standard. The assay and reference material are specific to DYSPORT. One unit of DYSPORT corresponds to the calculated median lethal intraperitoneal dose (LD50) in mice. Due to specific details of the assay system, such as vehicle, dilution scheme and laboratory protocols, Units of biological activity of DYSPORT cannot be converted into Units of any other botulinum toxin or any toxin assessed with any other specific assay method.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
DYSPORT inhibits release of the neurotransmitter, acetylcholine, from peripheral cholinergic nerve endings. Toxin activity occurs in the following sequence: Toxin heavy chain mediated binding to specific surface receptors on nerve endings, internalization of the toxin by receptor mediated endocytosis, pH-induced translocation of the toxin light chain to the cell cytosol and cleavage of SNAP25 leading to intracellular blockage of neurotransmitter exocytosis into the neuromuscular junction. This accounts for the therapeutic utility of the toxin in diseases characterized by excessive efferent activity in motor nerves.
Recovery of transmission occurs gradually as the neuromuscular junction recovers from SNAP25 cleavage and as new nerve endings are formed.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Impairment of Fertility
In a fertility and early embryonic development study in rats in which either males (2.9, 7.2, 14.5 or 29 Units/kg) or females (7.4, 19.7, 39.4 or 78.8 Units/kg) received weekly intramuscular injections prior to and after mating, dose-related increases in pre-implantation loss and reduced numbers of corpora lutea were noted in treated females. Failure to mate was observed in males that received the high dose. The no-effect dose for effects on fertility was 7.4 Units/kg in females and 14.5 Units/kg in males (approximately one-half and equal to, respectively, the maximum recommended human dose of 1000 Units on a body weight basis).
-
14 CLINICAL STUDIES
14.1 Cervical Dystonia
The efficacy of DYSPORT was evaluated in two randomized, double-blind, placebo-controlled, single-dose, parallel-group studies in treatment-naive cervical dystonia patients. The principal analyses from these trials provide the primary demonstration of efficacy involving 252 patients (121 on DYSPORT, 131 on placebo) with 36% male and 64% female. Ninety-nine percent of the patients were Caucasian.
In both placebo-controlled studies (Study 1 and Study 2), a dose of 500 Units of DYSPORT was given by intramuscular injection divided among two to four affected muscles. These studies were followed by long-term open-label extensions that allowed titration in 250 Unit steps to doses in a range of 250 to 1000 Units, after the initial dose of 500 Units. In the extension studies, re-treatment was determined by clinical need after a minimum of 12 weeks. The median time to re-treatment was 14 weeks and 18 weeks for the 75th percentile.
The primary assessment of efficacy was based on the total Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) change from baseline at Week 4 for both studies. The scale evaluates the severity of dystonia, patient-perceived disability from dystonia, and pain. The adjusted mean change from baseline in the TWSTRS total score was statistically significantly greater for the DYSPORT group than the placebo group at Week 4 in both studies (see Table 14).
Table 14: TWSTRS Total Score Efficacy Outcome from the Phase 3 Cervical Dystonia Studies Intent to Treat Population Study 1 Study 2 DYSPORT
500 Units
N=55Placebo
N=61DYSPORT
500 Units
N=37Placebo
N=43Baseline (Week 0) Mean (SD) 43.8 (8.0) 45.8 (8.9) 45.1 (8.7) 46.2 (9.4) Week 4 Mean (SD) 30.0 (12.7) 40.2 (11.8) 35.2 (13.8) 42.4 (12.2) Change from Baseline* -15.6 (2.0) -6.7 (2.0) -9.6 (2.0) -3.7 (1.8) Treatment difference -8.9† -5.9† 95% confidence interval [-12.9 to -4.7] [-10.6 to -1.3] Week 8
Mean (SD) 29.3 (11.0) 39.6 (13.5) Change from Baseline* -14.7 (2.0) -5.9 (2.0) Treatment difference -8.8† 95% confidence interval [-12.9 to -4.7] Analyses by gender, weight, geographic region, underlying pain, cervical dystonia severity at baseline and history of treatment with botulinum toxin did not show any meaningful differences between groups.
Table 15 indicates the average DYSPORT dose, and percentage of total dose, injected into specific muscles in the pivotal clinical trials.
Table 15: DYSPORT 500 Units starting dose (units and % of the total dose) by Unilateral Muscle Injected During Double-Blind Pivotal Phase 3 studies 2 and 1 Combined Number of patients injected per muscle* DYSPORT Dose Injected Percentage of the total DYSPORT Dose Injected Median
[DYSPORT Units]
(min, max)75th percentile
[DYSPORT Units]Median
[%]
(min, max)75th percentile
[%]- *
- Total number of patients in combined studies 2 and 1 who received initial treatment = 121.
Sternocleidomastoid 90 125 Units
(50, 350)150 Units 26.5 %
(10, 70)30.0 % Splenius capitis 85 200 Units
(75, 450)250 Units 40.0 %
(15, 90)50.0 % Trapezius 50 102.6 Units
(50, 300)150 Units 20.6 %
(10, 60)30.0 % Levator scapulae 35 105.3 Units
(50, 200)125 Units 21.1 %
(10, 40)25.0 % Scalenus (medius and anterior) 26 115.5 Units
(50, 300)150 Units 23.1 %
(10, 60)30.0 % Semispinalis capitis 21 131.6 Units
(50, 250)175 Units 29.4 %
(10, 50)35.0 % Longissimus 3 150 Units
(100, 200)200 Units 30.0 %
(20, 40)40.0 % 14.2 Glabellar Lines
Three double-blind, randomized, placebo-controlled, clinical studies evaluated the efficacy of DYSPORT for use in the temporary improvement of the appearance of moderate to severe glabellar lines. These three studies enrolled healthy adults (ages 19-75) with glabellar lines of at least moderate severity at maximum frown. Subjects were excluded if they had marked ptosis, deep dermal scarring, or a substantial inability to lessen glabellar lines, even by physically spreading them apart. The subjects in these studies received either DYSPORT or placebo. The total dose was delivered in equally divided aliquots to specified injection sites (see Figure 1).
Investigators and subjects assessed efficacy at maximum frown by using a 4-point scale (none, mild, moderate, severe).
Overall treatment success was defined as post-treatment glabellar line severity of none or mild with at least 2 grade improvement from baseline for the combined investigator and subject assessments (composite assessment) on Day 30 (see Table 16). Additional endpoints for each of the studies were post-treatment glabellar line severity of none or mild with at least a 1 grade improvement from baseline for the separate investigator and subject assessments on Day 30.
After completion of the randomized studies, subjects were offered participation in a two-year, open-label re-treatment study to assess the safety of multiple treatments.
Table 16: Treatment Success at Day 30 (None or Mild with at least 2 Grade Improvement from Baseline at Maximum Frown for the combined Investigator and Subject Assessments (Composite)) 2 Grade Improvement Study DYSPORT
n/N (%)Placebo
n/N (%)GL-1 58/105 (55%) 0/53 (0%) GL-2 37/71 (52%) 0/71 (0%) GL-3 120/200 (60%) 0/100 (0%) Treatment with DYSPORT reduced the severity of glabellar lines for up to four months.
Study GL-1
Study GL-1 was a single-dose, double-blind, multicenter, randomized, placebo-controlled study in which 158 previously untreated subjects received either placebo or 50 Units of DYSPORT, administered in five aliquots of 10 Units (see Figure 1). Subjects were followed for 180 days. The mean age was 43 years; most of the subjects were women (85%), and predominantly Caucasian (49%) or Hispanic (47%). At Day 30, 55% of DYSPORT-treated subjects achieved treatment success: a composite 2 grade improvement of glabellar line severity at maximum frown (see Table 16).
In study GL-1, the reduction of glabellar line severity at maximum frown was greater at Day 30 in the DYSPORT group compared to the placebo group as assessed by both Investigators and subjects (see Table 17).
Table 17: GL-1: Investigators' and Subjects' Assessment of Glabellar Line Severity at Maximum Frown Using a 4-point Scale (% and Number of Subjects with Severity of None or Mild) Investigators' Assessment Subjects' Assessment Day DYSPORT
N=105Placebo
N=53DYSPORT
N=105Placebo
N=5314 90%
9517%
977%
819%
530 88%
924%
274%
789%
560 64%
672%
160%
636%
390 43%
456%
336%
386%
3120 23%
244%
219%
206%
3150 9%
92%
18%
84%
2180 6%
60%
07%
78%
4Study GL-2
Study GL-2 was a repeat-dose, double-blind, multicenter, placebo-controlled, randomized study. The study was initiated with two or three open-label treatment cycles of 50 Units of DYSPORT administered in five aliquots of 10 Units DYSPORT (see Figure 1). After the open-label treatments, subjects were randomized to receive either placebo or 50 Units of DYSPORT. Subjects could have received up to four treatments through the course of the study. Efficacy was assessed in the final randomized treatment cycle. The study enrolled 311 subjects into the first treatment cycle and 142 subjects were randomized into the final treatment cycle. Overall, the mean age was 47 years; most of the subjects were women (86%) and predominantly Caucasian (80%).
At Day 30, 52% of DYSPORT-treated subjects achieved treatment success: a composite 2 grade improvement of glabellar line severity at maximum frown (see Table 16).
The proportion of responders in the final treatment cycle was comparable to the proportion of responders in all prior treatment cycles.
After the final repeat treatment with DYSPORT, the reduction of glabellar line severity at maximum frown was greater at Day 30 in the DYSPORT group compared to the placebo group as assessed by both Investigators and subjects (see Table 18).
Table 18: GL-2: Investigators' and Subjects' Assessments of Glabellar Line Severity at Maximum Frown Using a 4-point Scale (% and Number of Subjects with Severity of None or Mild) Investigators' Assessment Subjects' Assessment Day DYSPORT
N=71Placebo
N=71DYSPORT
N=71Placebo
N=7130 85%
604%
379%
561%
1Study GL-3
Study GL-3 was a single-dose, double-blind, multicenter, randomized, placebo-controlled study in which 300 previously untreated subjects received either placebo or 50 Units of DYSPORT, administered in five aliquots of 10 Units (see Figure 1). Subjects were followed for 150 days. The mean age was 44 years; most of the subjects were women (87%), and predominantly Caucasian (75%) or Hispanic (18%).
At Day 30, 60% of DYSPORT-treated subjects achieved treatment success: a composite 2 grade improvement of glabellar line severity at maximum frown (see Table 16).
In study GL-3, the reduction of glabellar line severity at maximum frown was greater at Day 30 in the DYSPORT group compared to the placebo group as assessed by both Investigators and subjects (see Table 19).
Table 19. GL-3: Investigators' and Subjects' Assessment of Glabellar Line Severity at Maximum Frown Using a 4-point Scale (% and Number of Subjects with Severity of None or Mild) Investigators' Assessment Subjects' Assessment Day DYSPORT
N=200Placebo
N=100DYSPORT
N=200Placebo
N=10014 83%
1665%
583%
1652%
230 86%
1710%
082%
1632%
260 75%
1501%
165%
1304%
490 51%
1021%
146%
912%
2120 29%
581%
131%
613%
3150 16%
321%
116%
313%
314.3 Spasticity in Adults
Upper Limb Spasticity
The efficacy and safety of DYSPORT for the treatment of upper limb spasticity in adults was evaluated in a randomized, multicenter, double-blind, placebo-controlled study that included 238 patients (159 DYSPORT and 79 placebo) with upper limb spasticity (Modified Ashworth Scale (MAS) score ≥2 in the primary targeted muscle group for toxin-naive patients or MAS score ≥3 in the primary targeted muscle group for toxin non-naive patients at least 4 months after the last botulinum toxin injection, of any serotype) who were at least 6 months post-stroke or post-traumatic brain injury. The median age of the patients in this study was 55 years (range 18 to 78 years), 64% were male, and 86% were Caucasian.
DYSPORT 500 Units (N=80), DYSPORT 1000 Units (N=79), or placebo (N=79) was injected intramuscularly into the affected upper limb muscles. After injection of the primary targeted muscle groups (PTMG), the remainder of the dose was injected into at least two additional upper limb muscles determined by the patient's individual presentation. Table 20 provides the mean and range of DYSPORT doses injected and the number of injections into specific muscles of the upper limb.
Table 20: DYSPORT Dose Injected and Number of Injections per Muscle in Adults with Upper Limb Spasticity Muscle DYSPORT Treatment Group Number of Patients Mean
DYSPORT Units Injected
(Min, Max)Number of Injection Sites
Median, [Q1 ; Q3]- *
- Primary Targeted Muscle Group
Flexor digitorum profundus (FDP)* 500 U 54 93.5 Units (50 to 100) 1, [1 ; 2] 1000 U 65 195.5 Units (100 to 300) 2, [1 ; 2] Flexor digitorum superficialis (FDS)* 500 U 63 95.4 Units (50 to 100) 2, [1 ; 2] 1000 U 73 196.8 Units (100 to 300) 2, [1 ; 2] Flexor carpi radialis (FCR)* 500 U 57 92.2 Units (25 to 100) 1, [1 ; 2] 1000 U 57 178.1 Units (80 to 300) 1, [1 ; 2] Flexor carpi ulnaris (FCU)* 500 U 47 89.9 Units (25 to 180) 1, [1 ; 2] 1000 U 49 171.2 Units (80 to 200) 1, [1 ; 2] Brachialis* 500 U 60 148.5 Units (50 to 200) 2, [1 ; 2] 1000 U 43 321.4 Units (100 to 400) 2, [2 ; 2] Brachioradialis* 500 U 42 88.3 Units (50 to 200) 1, [1 ; 2] 1000 U 28 172.1 Units (50 to 200) 1, [1 ; 2] Biceps Brachii (BB) 500 U 28 106.4 Units (50 to 200) 2, [1 ; 2] 1000 U 19 207.4 Units (100 to 400) 2, [1 ; 2] Pronator Teres 500 U 14 81.8 Units (45 to 200) 1, [1 ; 1] 1000 U 30 157.3 Units (80 to 200) 1, [1 ; 1] The co-primary efficacy variables were muscle tone assessed by the MAS at the primary targeted muscle group at Week 4 and the Physician Global Assessment (PGA; ranges from –4 = markedly worse to +4= markedly improved) at Week 4 (see Table 21).
Table 21: Primary Endpoints (PTMG MAS and PGA) and MAS by Muscle Group at Week 4 in Adults with Upper Limb Spasticity Placebo
(N=79)DYSPORT (500 Units)
(N=80)(1000 Units)
(N=79)LS= Least Square; - *
- p≤0.05
LS Mean Change from Baseline in PTMG Muscle Tone on the MAS -0.3 -1.2* -1.4* LS Mean PGA of Response to Treatment 0.7 1.4* 1.8* LS Mean Change from Baseline in Wrist Flexor Muscle Tone on the MAS -0.3
(n=54)-1.4
(n=57)-1.6
(n=58)LS Mean Change from Baseline in Finger Flexor Muscle Tone on the MAS -0.3
(n=70)-0.9
(n=66)-1.2
(n=73)LS Mean Change from Baseline in Elbow Flexor Muscle Tone on the MAS -0.3
(n=56)-1.0
(n=61)-1.2
(n=48)Lower Limb Spasticity
The efficacy of DYSPORT for the treatment of lower limb spasticity was evaluated in a randomized, multicenter, double-blind, placebo-controlled study that included 381 patients (253 DYSPORT and 128 placebo). Patients had lower limb spasticity (Modified Ashworth Scale (MAS) score ≥2 in the affected ankle joint for toxin-naive patients, or MAS score ≥3 in the affected ankle joint for toxin non-naive patients) and were at least 6 months post-stroke or post-traumatic brain injury.
Table 22 provides the median DYSPORT doses injected and the number of injections into specific muscles of the lower limb as reported in the double-blind study. In the study, the gastrocnemius and soleus muscles, and at least one additional lower limb muscle were injected, according to the clinical presentation.
Table 22: DYSPORT Dose Injected and Number of Injections per Muscle in the Lower Limb - Median for the 1000 Unit and 1500 Unit Dose Groups Injected Muscle DYSPORT Units Injected Number of Injection Sites Gastrocnemius
Lateral100 Units to 150 Units 1 Medial 100 Units to 150 Units 1 Soleus 333 Units to 500 Units 3 Tibialis posterior 200 Units to 300 Units 2 Flexor digitorum longus 133 Units to 200 Units 1 to 2 Flexor hallucis longus 67 Units to 200 Units 1 The primary efficacy variable was muscle tone assessed by the MAS at the ankle joint at Week 4. The first secondary endpoint was the Physician Global Assessment at Week 4 (see Table 23).
Table 23: Primary Endpoint Change in MAS and the First Secondary Endpoint PGA at Week 4 in Adults with Lower Limb Spasticity LS Mean Change from Baseline on the Modified Ashworth Scale DYSPORT
1000 Units
(N=125)DYSPORT
1500 Units
(N=128)Placebo
(N=128)- *
- p<0.05
Week 4 -0.6 -0.8* -0.5 LS Mean Physician Global Assessment Score Investigator Week 4 0.9 0.9 0.7 14.4 Spasticity in Pediatric Patients
Upper Limb Spasticity in Pediatric Patients
The efficacy of DYSPORT for the treatment of upper limb spasticity in pediatric patients 2 to 17 years of age was evaluated in a double-blind, low-dose controlled, multicenter study (NCT02106351). A total of 208 patients with spasticity because of cerebral palsy who were toxin naive or non-naive (66% had prior treatment with a botulinum toxin), weighed at least 10 kgs, and had a baseline Modified Ashworth Score (MAS) of grade 2 or greater (99% patients) at the primary targeted muscle groups (PTMG) were enrolled in the modified Intention to Treat population (mITT). Patients received DYSPORT 16 Units/kg (n=70), DYSPORT 8 Units/kg (n=69), or DYSPORT 2 Units/kg (n=69) injected into the upper limb. The elbow flexors and wrist flexors respectively were the PTMG in 57% and in 43% of patients. The median age of the patients in this study was 9 years (range 2 to 17 years; 57% were between 2 and 9 years of age); 60% of patients were male, and 75% were White.
The primary efficacy endpoint was the mean change from baseline in MAS in the PTMG at Week 6 (see Table 24). The secondary efficacy endpoint was the mean Physician Global Assessment (PGA) score assessed at Week 6 (Table 25). Although PGA scores numerically favored DYSPORT treatment over the low-dose control, the difference was not statistically significant.
Table 24: Modified Ashworth Scale Score in the PTMG Change from Baseline at Week 6 in Pediatric Patients with Upper Limb Spasticity (mITT Population) Control Group Treatment Groups DYSPORT
2 U/kg
(N=69)DYSPORT
8 U/kg
(N=69)DYSPORT
16 U/kg
(N=70)Baseline Mean (SD) 3.1 (0.3) 3.1 (0.3) 3.1 (0.5) Week 6 LS* mean change from baseline in PTMG† on MAS -1.6 -2.0 -2.3 Difference from control in LS* means -0.4 -0.7 p-value ‡ 0.0118§ <0.0001 Week 16 LS mean change from baseline in PTMG† on MAS -0.9 -1.2 -1.5 Difference from control in LS* means -0.3§ -0.6§ Table 25: Physician Global Assessment of Treatment Response at Week 6 in Pediatric Patients with Upper Limb Spasticity (mITT Population) Control Group Treatment Groups Dysport
2 U/kg
(N=68)Dysport
8 U/kg
(N=69)Dysport
16 U/kg
(N=70)Week 6 Mean score (SD) 1.7 (0.9) 2.0 (0.9) 2.0 (0.9) LS* mean in PGA 1.8 2.0 2.0 Difference from control in LS* means 0.2 0.2 p-value † 0.2043 0.1880 Week 16 Mean score (SD) 1.7 (1.0) 1.6 (1.1) 1.9 (1.2) LS* mean in PGA 1.8 1.7 1.9 Difference from control in LS* means -0.1 0.1 p-value † 0.7001 0.4041 Lower Limb Spasticity in Pediatric Patients
The efficacy of DYSPORT for the treatment of lower limb spasticity in patients 2 to 17 years of age was evaluated in a double-blind, placebo-controlled, multicenter study . A total of 235 patients with cerebral palsy causing dynamic equinus foot deformity who were toxin-naive or non-naive and had a Modified Ashworth Score (MAS) of grade 2 or greater at the ankle plantar flexors were enrolled. Patients received DYSPORT 10 Units/kg/leg (n=79), DYSPORT 15 Units/kg/leg (n=79) or placebo (n=77) injected into the gastrocnemius and soleus muscles (see Table 27). Forty-one percent of patients (n=66) were treated bilaterally and received a total lower limb DYSPORT dose of either 20 Units/kg (n=37) or 30 Units/kg (n=29). The median age of the patients in this study was 5 years (range 2 to 17 years); 60% of patients were male, and 73% were Caucasian.
The primary efficacy endpoint was the mean change from baseline in MAS in ankle plantar flexor at Week 4; a co-primary endpoint was the mean Physician's Global Assessment (PGA) score at Week 4 (see Table 26).
Table 26: MAS and PGA Change from Baseline at Week 4 in Pediatric Patients with Lower Limb Spasticity (ITT Population) Placebo
(N=77)DYSPORT
10 Units/kg/leg
(N=79)DYSPORT
15 Units/kg/leg
(N=79)LS=Least Square - *
- p<0.05
LS Mean Change from Baseline in Ankle plantar flexor Muscle Tone on the MAS Week 4 -0.5 -0.9* -1.0* Week 12 -0.5 -0.8* -1.0* LS Mean PGA of Response to Treatment Week 4 0.7 1.5* 1.5* Week 12 0.4 0.8* 1.0* -
16 HOW SUPPLIED/STORAGE AND HANDLING
DYSPORT® (abobotulinumtoxinA) for injection is a sterile, lyophilized powder supplied in a single-dose, glass vial. Unopened vials of DYSPORT must be stored refrigerated between 2°C to 8°C (36°F to 46°F). Protect from light.
Do not use after the expiration date on the vial. All vials, including expired vials, or equipment used with DYSPORT should be disposed of carefully as is done with all medical waste.
DYSPORT contains a unique hologram on the carton. If you do not see the hologram, do not use the product. Instead contact 855-463-5127.
Cervical Dystonia, Spasticity in Adults and Pediatric Patients
500 Unit Vial
Each vial contains 500 Units of freeze-dried abobotulinumtoxinA.
Box containing 1 vial—NDC 15054-0500-1
Box containing 2 vials—NDC 15054-0500-2300 Unit Vial
Each vial contains 300 Units of freeze-dried abobotulinumtoxinA.
Box containing 1 vial—NDC 15054-0530-6 -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Swallowing, Speaking, or Breathing Difficulties, or Other Unusual Symptoms
Advise patients to inform their doctor or pharmacist if they develop any unusual symptoms (including difficulty with swallowing, speaking or breathing), or if any known symptom persists or worsens [see Warnings and Precautions (5.1, 5.4)].
Dry Eye with the Treatment of Glabellar Lines
Inform patients that DYSPORT injection may cause eye dryness [see Warnings and Precautions (5.6)]. Advise patients to report symptoms of eye dryness (e.g., eye pain, eye irritation, photosensitivity, or changes in vision) to their doctor.
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised 9/2023 MEDICATION GUIDE
DYSPORT® (DIS-port)
(abobotulinumtoxinA)
for InjectionWhat is the most important information I should know about DYSPORT?
DYSPORT may cause serious side effects that can be life threatening including:
- Problems breathing or swallowing
- Spread of toxin effects
These problems can happen within hours, or days to weeks after an injection of DYSPORT. Call your doctor or get medical help right away if you have any of these problems after treatment with DYSPORT:
1. Problems swallowing, speaking, or breathing. These problems can happen within hours, or days to weeks after an injection of DYSPORT usually because the muscles that you use to breathe and swallow can become weak after the injection. Death can happen as a complication if you have severe problems with swallowing or breathing after treatment with DYSPORT.
- People with certain breathing problems may need to use muscles in their neck to help them breathe. These patients may be at greater risk for serious breathing problems with DYSPORT.
- Swallowing problems may last for several weeks. People who cannot swallow well may need a feeding tube to receive food and water. If swallowing problems are severe, food or liquids may go into your lungs. People who already have swallowing or breathing problems before receiving DYSPORT have the highest risk of getting these problems.
2. Spread of toxin effects. In some cases, the effect of botulinum toxin may affect areas of the body away from the injection site and cause symptoms of a serious condition called botulism. The symptoms of botulism include:
- loss of strength and muscle weakness all over the body
- blurred vision and drooping eyelids
- trouble saying words clearly (dysarthria)
- trouble breathing
- double vision
- hoarseness or change or loss of voice (dysphonia)
- loss of bladder control
- trouble swallowing
These symptoms can happen within hours, or days to weeks after you receive an injection of DYSPORT. These problems could make it unsafe for you to drive a car or do other dangerous activities. See "What should I avoid while receiving DYSPORT?"
What is DYSPORT?
DYSPORT is a prescription medicine that is injected into muscles and used:
- to treat cervical dystonia (CD) in adults
- to improve the look of moderate to severe frown lines between the eyebrows (glabellar lines) in adults younger than 65 years of age for a short period of time (temporary)
- to treat increased muscle stiffness in people 2 years of age and older with spasticity
It is not known whether DYSPORT is safe or effective in people younger than:
- 18 years of age for the treatment of cervical dystonia
- 18 years of age for the treatment of glabellar lines
- 2 years of age for the treatment of spasticity
It is not known whether DYSPORT is safe or effective for the treatment of other wrinkles.
Who should not take DYSPORT?
Do not take DYSPORT if you:
- are allergic to DYSPORT or any of the ingredients in DYSPORT. See the end of this Medication Guide for a list of ingredients in DYSPORT
- are allergic to cow's milk protein
- had an allergic reaction to any other botulinum toxin product such as Myobloc® (rimabotulinumtoxinB), Botox® (onabotulinumtoxinA), or Xeomin® (incobotulinumtoxinA)
- have a skin infection at the planned injection site
What should I tell my doctor before taking DYSPORT?
Tell your doctor about all your medical conditions, including if you:
- have a disease that affects your muscles and nerves (such as amyotrophic lateral sclerosis [ALS or Lou Gehrig's disease], myasthenia gravis or Lambert-Eaton syndrome). See "What is the most important information I should know about DYSPORT?"
- have allergies to any botulinum toxin product
- had any side effect from any botulinum toxin product in the past
- have or have had a breathing problem, such as asthma or emphysema
- have or have had swallowing problems
- have or have had bleeding problems
- have diabetes
- have or have had a slow heart beat or other problem with your heart rate or rhythm
- have plans to have surgery
- had surgery on your face
- have weakness in or near your muscles being treated
- have drooping eyelids
- experienced dry eye with previous use of botulinum toxin products
- have any other change in the way your face normally looks
- are pregnant or plan to become pregnant. It is not known if DYSPORT can harm your unborn baby
- are breast-feeding or planning to breast-feed. It is not known if DYSPORT passes into breast milk
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal products. Using DYSPORT with certain other medicines may cause serious side effects. Do not start any new medicines until you have told your doctor that you have received DYSPORT in the past.
Especially tell your doctor if you:
- have received any other botulinum toxin product in the last four months
- have received injections of botulinum toxin, such as Myobloc® (rimabotulinumtoxinB), Botox® (onabotulinumtoxinA) or Xeomin® (incobotulinumtoxinA) in the past; be sure your doctor knows exactly which product you received
- have recently received an antibiotic by injection
- take muscle relaxants
- take an allergy or cold medicine
- take a sleep medicine
Ask your doctor if you are not sure if your medicine is one that is listed above.
Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist each time you get a new medicine.
How will I receive DYSPORT?
- DYSPORT is an injection that your doctor will give you
- DYSPORT is injected into the affected muscles
- If you are an adult, your doctor may give you another dose of DYSPORT after 12 weeks or longer, if it is needed
- If you are an adult being treated for CD or spasticity or you are a child (2 to 17 years of age) being treated for spasticity, your doctor may change your dose of DYSPORT until you and your doctor find the best dose for you. Children should not be retreated sooner than every 12-16 weeks
- The dose of DYSPORT is not the same as the dose of any other botulinum toxin product
What should I avoid while receiving DYSPORT?
DYSPORT may cause loss of strength or general muscle weakness, blurred vision, or drooping eyelids within hours to weeks of taking DYSPORT. If this happens, do not drive a car, operate machinery, or do other dangerous activities. See "What is the most important information I should know about DYSPORT?"
What are the possible side effects of DYSPORT?
DYSPORT can cause serious side effects. See "What is the most important information I should know about DYSPORT?"
The most common side effects of DYSPORT in people with cervical dystonia include:
- muscle weakness
- dry mouth
- feeling of tiredness
- muscle pain
- problems speaking
- eye problems
- difficulty swallowing
- injection site pain or discomfort
- headache
The most common side effects of DYSPORT in people with glabellar lines include:
- stuffy or runny nose and sore throat
- injection site pain
- upper respiratory infection
- blood in urine
- headache
- injection site reaction
- swelling of eyelids
- drooping eyelids
- sinus infection
- nausea
The most common side effect of DYSPORT in adults with upper limb spasticity include:
- muscle weakness
The most common side effects of DYSPORT in adults with lower limb spasticity include:
- muscle weakness
- pain in your arms or legs
- fall
The most common side effects of DYSPORT in children (2 to 17 years of age) with upper limb spasticity include:
- upper respiratory tract infection
- sore throat
The most common side effects of DYSPORT in children (2 to 17 years of age) with lower limb spasticity include:
- stuffy or runny nose and sore throat
- cough
- fever
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of DYSPORT. For more information, ask your doctor or pharmacist.
Tell your doctor if you have dry eye or changes in vision following use of DYSPORT.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about DYSPORT:
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide.
This Medication Guide summarizes the most important information about DYSPORT. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about DYSPORT that is written for healthcare professionals.
What are the ingredients in DYSPORT?
Active ingredient: (botulinum toxin Type A)
Inactive ingredients: human albumin and lactose. DYSPORT may contain cow's milk protein.
Distributed by: Ipsen Biopharmaceuticals, Inc. Cambridge, MA 02142 and Galderma Laboratories, L.P. Dallas, TX 75201; Manufactured by: Ipsen Biopharm Ltd., Wrexham, LL13 9UF, UK U.S. License No. 1787
For more information about DYSPORT, call 855-463-5127 or go to www.dysport.com or www.DysportUSA.com.
DYSPORT is a registered trademark of Ipsen Biopharm Limited. Botox, Xeomin and Myobloc are registered trademarks of their respective owners.
© 2023. All rights reserved.
- PRINCIPAL DISPLAY PANEL - 500 Units Vial Carton
- PRINCIPAL DISPLAY PANEL - 300 Units Vial Carton
-
INGREDIENTS AND APPEARANCE
DYSPORT
botulinum toxin type a injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:15054-0500 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Botulinum Toxin Type A (UNII: E211KPY694) (Botulinum Toxin Type A - UNII:E211KPY694) Botulinum Toxin Type A 500 U Inactive Ingredients Ingredient Name Strength albumin human (UNII: ZIF514RVZR) lactose monohydrate (UNII: EWQ57Q8I5X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:15054-0500-1 1 in 1 CARTON 11/02/2009 1 1 in 1 VIAL; Type 0: Not a Combination Product 2 NDC:15054-0500-2 2 in 1 CARTON 11/02/2009 2 1 in 1 VIAL; Type 0: Not a Combination Product 3 NDC:15054-0500-9 1 in 1 CARTON 11/02/2009 3 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125274 11/02/2009 DYSPORT
botulinum toxin type a injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:15054-0530 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Botulinum Toxin Type A (UNII: E211KPY694) (Botulinum Toxin Type A - UNII:E211KPY694) Botulinum Toxin Type A 300 U Inactive Ingredients Ingredient Name Strength albumin human (UNII: ZIF514RVZR) lactose monohydrate (UNII: EWQ57Q8I5X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:15054-0530-6 1 in 1 CARTON 11/02/2009 1 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125274 11/02/2009 Labeler - Ipsen Biopharmaceuticals, Inc. (118461578) Establishment Name Address ID/FEI Business Operations IPSEN BIOPHARM LTD 365113203 MANUFACTURE(15054-0500, 15054-0530)