Label: FUL-GLO- fluorescein sodium strip
-
Contains inactivated NDC Code(s)
NDC Code(s): 17478-404-01 - Packager: Akorn
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 12, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
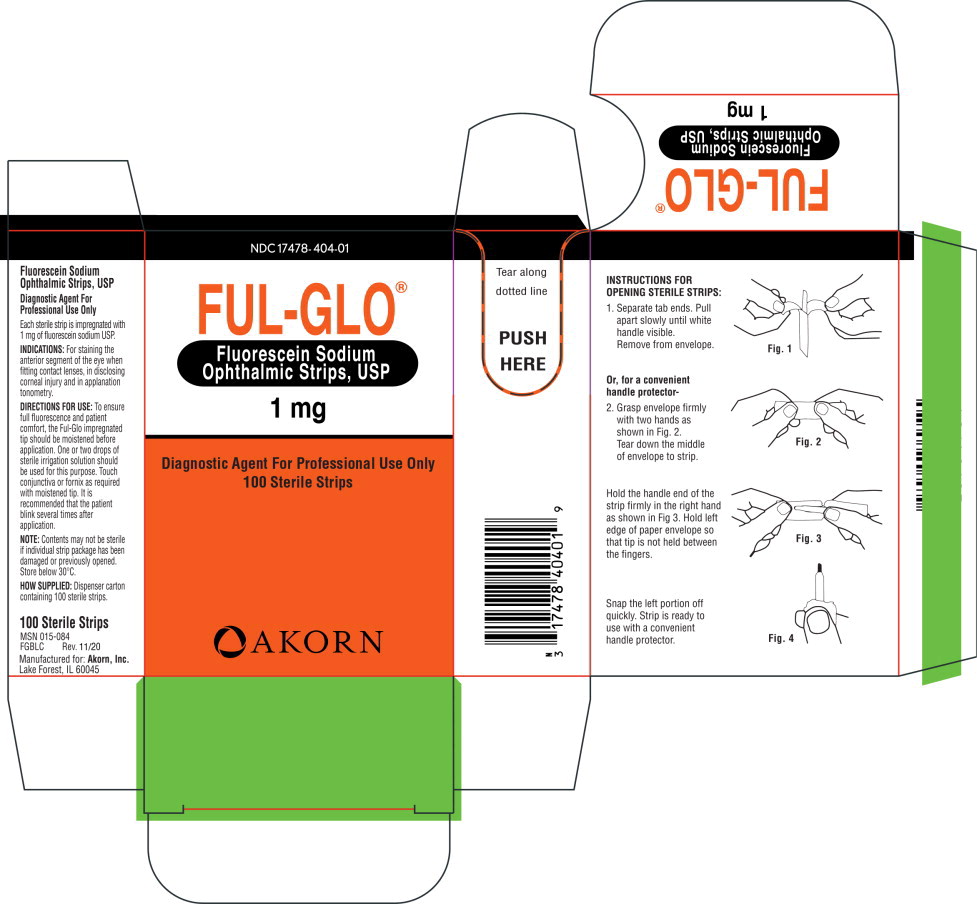
- INDICATIONS:
-
DIRECTIONS FOR USE:
To ensure full fluorescence and patient comfort, the Ful-Glo impregnated tip should be moistened before application. One or two drops of sterile irrigation solution should be used for this purpose. Touch conjunctiva or fornix as required with moistened tip. It is recommended that the patient blink several times after application.
NOTE: Contents may not be sterile if individual strip package has been damaged or previously opened. Store below 30°C.
- HOW SUPPLIED:
-
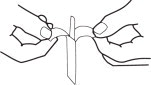
INSTRUCTIONS FOR OPENING STERILE STRIPS:
1. Separate tab ends. Pull apart slowly until white handle visible. Remove from envelope.
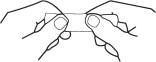
Or, for a convenient handle protector-
2. Grasp envelope firmly with two hands as shown in Fig. 2. Tear down the middle of envelope to strip.
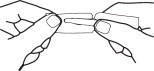
Hold the handle end of the strip firmly in the right hand as shown in Fig 3. Hold left edge of paper envelope so that tip is not held between the fingers.
Snap the left portion off quickly. Strip is ready to use with a convenient handle protector.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FUL-GLO
fluorescein sodium stripProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:17478-404 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fluorescein Sodium (UNII: 93X55PE38X) (Fluorescein - UNII:TPY09G7XIR) Fluorescein Sodium 1 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17478-404-01 100 in 1 CARTON 06/01/2004 1 1 in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 06/01/2004 Labeler - Akorn (117693100)