Label: OGIVRI- trastuzumab-dkst kit
OGIVRI- trastuzumab-dkst injection, powder, lyophilized, for solution
-
NDC Code(s):
83257-001-11,
83257-002-11,
83257-003-01,
83257-003-11, view more83257-004-12
- Packager: Biocon Biologics Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated August 30, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OGIVRI safely and effectively. See full prescribing information for OGIVRI.
OGIVRI® (trastuzumab-dkst) for injection, for intravenous use
Initial U.S. Approval: 2017
OGIVRI® (trastuzumab-dkst) is biosimilar* to HERCEPTIN (trastuzumab).WARNING: CARDIOMYOPATHY, INFUSION REACTIONS, EMBRYO-FETAL TOXICITY, and PULMONARY TOXICITY
See full prescribing information for complete boxed warning
Cardiomyopathy: Trastuzumab products can result in subclinical and clinical cardiac failure manifesting as CHF, and decreased LVEF, with greatest risk when administered concurrently with anthracyclines. Evaluate cardiac function prior to and during treatment. Discontinue Ogivri for cardiomyopathy. (2.5, 5.1)
Infusion Reactions, Pulmonary Toxicity: Discontinue Ogivri for anaphylaxis, angioedema, interstitial pneumonitis, or acute respiratory distress syndrome. (5.2, 5.4)
Embryo-Fetal Toxicity: Exposure to trastuzumab products during pregnancy can result in oligohydramnios, in some cases complicated by pulmonary hypoplasia and neonatal death. Advise patients of these risks and the need for effective contraception. (5.3, 8.1, 8.3)
RECENT MAJOR CHANGES
Dosage and Administration, Evaluation
and Testing Before Initiating Ogivri (2.1) 06/2024
INDICATIONS AND USAGE
Ogivri is a HER2/neu receptor antagonist indicated in adults for:
-
The treatment of HER2-overexpressing breast cancer. (1.1, 1.2)
-
The treatment of HER2-overexpressing metastatic gastric or gastroesophageal junction adenocarcinoma. (1.3)
Select patients for therapy based on an FDA-approved companion diagnostic for a trastuzumab product (1, 2.2).
DOSAGE AND ADMINISTRATION
For intravenous (IV) infusion only. Do not administer as an IV push or bolus. Ogivri has different dosage and administration instructions than subcutaneous trastuzumab products. (2.3)
Do not substitute Ogivri (trastuzumab-dkst) for or with ado-trastuzumab emtansine or fam-trastuzumab deruxtecan. (2.3)
Perform HER2 testing using FDA-approved tests by laboratories with demonstrated proficiency. (1, 2.2)
Adjuvant Treatment of HER2-Overexpressing Breast Cancer (2.2)
Administer at either:
-
Initial dose of 4 mg/kg over 90 minute IV infusion, then 2 mg/kg over 30 minute IV infusion weekly for 12 weeks (with paclitaxel or docetaxel) or 18 weeks (with docetaxel and carboplatin). One week after the last weekly dose of Ogivri, administer 6 mg/kg as an IV infusion over 30 to 90 minutes every three weeks to complete a total of 52 weeks of therapy, or
-
Initial dose of 8 mg/kg over 90 minutes IV infusion, then 6 mg/kg over 30 to 90 minutes IV infusion every three weeks for 52 weeks.
Metastatic HER2-Overexpressing Breast Cancer (2.3)
-
Initial dose of 4 mg/kg as a 90 minute IV infusion followed by subsequent weekly doses of 2 mg/kg as 30 minute IV infusions.
Metastatic HER2-Overexpressing Gastric Cancer (2.3)
-
Initial dose of 8 mg/kg over 90 minutes IV infusion, followed by 6 mg/kg over 30 to 90 minutes IV infusion every 3 weeks.
DOSAGE FORMS AND STRENGTHS
- •
- For Injection: 150 mg lyophilized powder in a single-dose vial for reconstitution (3)
- •
- For Injection: 420 mg lyophilized powder in a multiple-dose vial for reconstitution supplied with a separate vial containing 20 mL of Bacteriostatic Water for Injection, USP, to be used as a diluent. (3)
- •
- For Injection: 420 mg lyophilized powder in a multiple-dose vial for reconstitution. No diluent is provided. (3)
CONTRAINDICATIONS
- •
- None. (4)
ADVERSE REACTIONS
Adjuvant Breast Cancer
- •
- Most common adverse reactions (≥ 5%) are headache, diarrhea, nausea, and chills. (6.1)
Metastatic Breast Cancer
- •
- Most common adverse reactions (≥ 10%) are fever, chills, headache, infection, congestive heart failure, insomnia, cough, and rash. (6.1)
Metastatic Gastric Cancer
- •
- Most common adverse reactions (≥ 10%) are neutropenia, diarrhea, fatigue, anemia, stomatitis, weight loss, upper respiratory tract infections, fever, thrombocytopenia, mucosal inflammation, nasopharyngitis, and dysgeusia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Biocon Biologics at 1-833-986-1468 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Females and Males of Reproductive Potential: Verify the pregnancy status of females prior to initiation of Ogivri (8.3).
*Biosimilar means that the biological product is approved based on data demonstrating that it is highly similar to an FDA-approved biological product, known as a reference product, and that there are no clinically meaningful differences between the biosimilar product and the reference product. Biosimilarity of Ogivri has been demonstrated for the condition(s) of use (e.g. indication(s), dosing regimen(s)), strength(s), dosage form(s), and route(s) of administration described in its Full Prescribing Information.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2024
-
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CARDIOMYOPATHY, INFUSION REACTIONS, EMBRYO-FETAL TOXICITY, and PULMONARY TOXICITY
1 INDICATIONS AND USAGE
1.1 Adjuvant Breast Cancer
1.2 Metastatic Breast Cancer
1.3 Metastatic Gastric Cancer
2 DOSAGE AND ADMINISTRATION
2.1 Evaluation and Testing Before Initiating Ogivri
2.2 Patient Selection
2.3 Recommended Doses and Schedules
2.4 Important Dosing Considerations
2.5 Dosage Modifications for Adverse Reactions
2.6 Preparation Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cardiomyopathy
5.2 Infusion Reactions
5.3 Embryo-Fetal Toxicity
5.4 Pulmonary Toxicity
5.5 Exacerbation of Chemotherapy-Induced Neutropenia
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Adjuvant Breast Cancer
14.2 Metastatic Breast Cancer
14.3 Metastatic Gastric Cancer
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CARDIOMYOPATHY, INFUSION REACTIONS, EMBRYO-FETAL TOXICITY, and PULMONARY TOXICITY
Cardiomyopathy
Administration of trastuzumab products can result in sub-clinical and clinical cardiac failure. The incidence and severity was highest in patients receiving trastuzumab with anthracycline-containing chemotherapy regimens.
Evaluate left ventricular function in all patients prior to and during treatment with Ogivri. Discontinue Ogivri treatment in patients receiving adjuvant therapy and withhold Ogivri in patients with metastatic disease for clinically significant decrease in left ventricular function [see Dosage and Administration (2.5) and Warnings and Precautions (5.1)].
Infusion Reactions; Pulmonary Toxicity
Administration of trastuzumab products can result in serious and fatal infusion reactions and pulmonary toxicity. Symptoms usually occur during or within 24 hours of Ogivri administration.. Interrupt Ogivri infusion for dyspnea or clinically significant hypotension. Monitor patients until symptoms completely resolve. Discontinue Ogivri for anaphylaxis, angioedema, interstitial pneumonitis, or acute respiratory distress syndrome [see Warnings and Precautions (5.2, 5.4)].
Embryo-Fetal Toxicity
Exposure to trastuzumab products during pregnancy can result in oligohydramnios and oligohydramnios sequence manifesting as pulmonary hypoplasia, skeletal abnormalities, and neonatal death. Advise patients of these risks and the need for effective contraception [see Warnings and Precautions (5.3) and Use in Specific Populations (8.1, 8.3)].
-
1 INDICATIONS AND USAGE
1.1 Adjuvant Breast Cancer
Ogivri is indicated in adults for adjuvant treatment of HER2 overexpressing node positive or node negative (ER/PR negative or with one high risk feature [see Clinical Studies (14.1)]) breast cancer
-
as part of a treatment regimen consisting of doxorubicin, cyclophosphamide, and either paclitaxel or docetaxel
-
as part of a treatment regimen with docetaxel and carboplatin
-
as a single agent following multi-modality anthracycline based therapy.
Select patients for therapy based on an FDA-approved companion diagnostic for a trastuzumab product [see Dosage and Administration (2.2)].
1.2 Metastatic Breast Cancer
Ogivri is indicated in adults:
-
In combination with paclitaxel for first-line treatment of HER2-overexpressing metastatic breast cancer
-
As a single agent for treatment of HER2-overexpressing breast cancer in patients who have received one or more chemotherapy regimens for metastatic disease.
Select patients for therapy based on an FDA-approved companion diagnostic for a trastuzumab product [see Dosage and Administration (2.2)].
1.3 Metastatic Gastric Cancer
Ogivri is indicated, in combination with cisplatin and capecitabine or 5-fluorouracil, for the treatment of patients with HER2-overexpressing metastatic gastric or gastroesophageal junction adenocarcinoma who have not received prior treatment for metastatic disease.
Select patients for therapy based on an FDA-approved companion diagnostic for a trastuzumab product [see Dosage and Administration (2.2)].
-
-
2 DOSAGE AND ADMINISTRATION
2.1 Evaluation and Testing Before Initiating Ogivri
Assess left ventricular ejection fraction (LVEF) prior to initiation of Ogivri and at regular intervals during treatment. [see Boxed Warning, Dosage and Administration (2.5), Warnings and Precautions (5.1)].
Verify the pregnancy status of females of reproductive potential prior to the initiation of Ogivri [see Warnings and Precautions (5.3), Use in Specific Populations (8.1, 8.3)].
2.2 Patient Selection
Select patients based on HER2 protein overexpression or HER2 gene amplification in tumor specimens [see Indications and Usage (1) and Clinical Studies (14)]. Assessment of HER2 protein overexpression and HER2 gene amplification should be performed using FDA-approved tests specific for breast or gastric cancers by laboratories with demonstrated proficiency. Information on the FDA-approved tests for the detection of HER2 protein overexpression and HER2 gene amplification is available at: http://www.fda.gov/CompanionDiagnostics.
Assessment of HER2 protein overexpression and HER2 gene amplification in metastatic gastric cancer should be performed using FDA-approved tests specifically for gastric cancers due to differences in gastric vs. breast histopathology, including incomplete membrane staining and more frequent heterogeneous expression of HER2 seen in gastric cancers.
Improper assay performance, including use of suboptimally fixed tissue, failure to utilize specified reagents, deviation from specific assay instructions, and failure to include appropriate controls for assay validation, can lead to unreliable results.
2.3 Recommended Doses and Schedules
-
Ogivri is for intravenous infusion only. Do not administer as an intravenous push or bolus.
-
Ogivri has different dosage and administration instructions than subcutaneous trastuzumab products.
-
Do not mix Ogivri with other drugs.
-
Do not substitute Ogivri (trastuzumab-dkst) for or with ado-trastuzumab emtansine or fam-trastuzumab deruxtecan.
Adjuvant Treatment of Breast Cancer:
Administer according to one of the following doses and schedules for a total of 52 weeks of Ogivri therapy:
During and following paclitaxel, docetaxel, or docetaxel and carboplatin:
Initial dose of 4 mg/kg as an intravenous infusion over 90 minutes then at 2 mg/kg as an intravenous infusion over 30 minutes weekly during chemotherapy for the first 12 weeks (paclitaxel or docetaxel) or 18 weeks (docetaxel and carboplatin).
-
One week following the last weekly dose of Ogivri, administer Ogivri at 6 mg/kg as an intravenous infusion over 30 to 90 minutes every three weeks.
As a single agent within three weeks following completion of multi-modality, anthracycline-based chemotherapy regimens:
-
Initial dose at 8 mg/kg as an intravenous infusion over 90 minutes
-
Subsequent doses at 6 mg/kg as an intravenous infusion over 30 to 90 minutes every three weeks.
-
Extending adjuvant treatment beyond one year is not recommended [see Adverse Reactions (6.1)].
Metastatic Treatment, Breast Cancer:
-
Administer Ogivri, alone or in combination with paclitaxel, at an initial dose of 4 mg/kg as a 90-minute intravenous infusion followed by subsequent once weekly doses of 2 mg/kg as 30-minute intravenous infusions until disease progression.
Metastatic Gastric Cancer:
-
Administer Ogivri at an initial dose of 8 mg/kg as a 90 minute intravenous infusion followed by subsequent doses of 6 mg/kg as an intravenous infusion over 30 to 90 minutes every three weeks until disease progression.
2.4 Important Dosing Considerations
Missed Dose
If the patient has missed a dose of Ogivri by one week or less, then the usual maintenance dose (weekly schedule: 2 mg/kg; three-weekly schedule: 6 mg/kg) should be administered as soon as possible. Do not wait until the next planned cycle. Subsequent Ogivri maintenance doses should be administered 7 days or 21 days later according to the weekly or three-weekly schedules, respectively.
If the patient has missed a dose of Ogivri by more than one week, a re-loading dose of Ogivri should be administered over approximately 90 minutes (weekly schedule: 4 mg/kg; once every three-weekly schedule: 8 mg/kg) as soon as possible. Subsequent Ogivri maintenance doses (weekly schedule: 2 mg/kg; three-weekly schedule 6 mg/kg) should be administered 7 days or 21 days later according to the weekly or three-weekly schedules, respectively.
2.5 Dosage Modifications for Adverse Reactions
Infusion Reactions
[see Boxed Warning, Warnings and Precautions (5.2)]
-
Decrease the rate of infusion for mild or moderate infusion reactions
-
Interrupt the infusion in patients with dyspnea or clinically significant hypotension
-
Discontinue Ogivri for severe or life-threatening infusion reactions.
Cardiomyopathy
[see Boxed Warning, Warnings and Precautions (5.1)]
Assess left ventricular ejection fraction (LVEF) prior to initiation of Ogivri and at regular intervals during treatment. Withhold Ogivri dosing for at least 4 weeks for either of the following:
-
≥16% absolute decrease in LVEF from pre-treatment values
-
LVEF below institutional limits of normal and ≥ 10% absolute decrease in LVEF from pretreatment values.
Ogivri may be resumed if, within 4 to 8 weeks, the LVEF returns to normal limits and the absolute decrease from baseline is ≤15%.
Permanently discontinue Ogivri for a persistent (>8 weeks) LVEF decline or for suspension of Ogivri dosing on more than 3 occasions for cardiomyopathy.
2.6 Preparation Instructions
To prevent medication errors, it is important to check the vial labels to ensure that the drug being prepared and administered is Ogivri (trastuzumab-dkst) and not ado-trastuzumab emtansine or fam- trastuzumab deruxtecan.
420 mg Multiple-dose vial supplied with a separate vial containing 20 mL of Bacteriostatic Water for Injection, USP, to be used as diluent.
420 mg Multiple-dose vial drug only carton. No diluent is provided.
Reconstitution: Reconstitute each 420 mg vial of Ogivri with 20 mL of Bacteriostatic Water for Injection, USP (BWFI), containing 0.9% to 1.1% benzyl alcohol (not supplied for the 420 mg multiple-dose vial drug only carton) as a preservative to yield a multiple-dose solution containing 21 mg/mL trastuzumab-dkst that delivers 20 mL (420 mg trastuzumab-dkst). In patients with known hypersensitivity to benzyl alcohol, reconstitute with 20 mL of Sterile Water for Injection, USP (SWFI) without preservative to yield a one time use solution.
Use appropriate aseptic technique when performing the following reconstitution steps:
-
Using a sterile syringe, slowly inject the 20 mL of diluent into the vial containing the lyophilized powder of Ogivri, which has a cake-like appearance. The stream of diluent should be directed into the lyophilized cake. The reconstituted vial yields a solution for multiple-dose use, containing 21 mg/mL trastuzumab-dkst.
-
Swirl the vial gently to aid reconstitution. DO NOT SHAKE.
-
Slight foaming of the product may be present upon reconstitution. Allow the vial to stand undisturbed for approximately 5 minutes.
-
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Inspect visually for particulates and discoloration. The solution should be free of visible particulates, clear to slightly opalescent and colorless to pale yellow.
-
Store reconstituted Ogivri in the refrigerator at 2° to 8°C (36° to 46°F); discard unused Ogivri after 28 days. If Ogivri is reconstituted with SWFI without preservative, use immediately and discard any unused portion. Do not freeze.
Dilution:
Determine the dose (mg) of Ogivri [see Dosage and Administration (2.1)]. Calculate the volume of the 21 mg/mL reconstituted Ogivri solution needed.
-
Withdraw this amount from the vial and add it to an infusion bag containing 250 mL of 0.9% Sodium Chloride Injection, USP. DO NOT USE DEXTROSE (5%) SOLUTION.
-
Gently invert the bag to mix the solution.
-
The solution of Ogivri for infusion diluted in polyvinylchloride or polyethylene bags containing 0.9% Sodium Chloride Injection, USP, should be stored at 2° to 8°C (36° to 46°F) for no more than 24 hours prior to use. Do not freeze.
150 mg Single-dose vial
Reconstitution: Reconstitute each 150 mg vial of Ogivri with 7.4 mL of Sterile Water for Injection (SWFI) (not supplied) to yield a single-dose solution containing 21 mg/mL trastuzumab-dkst that delivers 7.15 mL (150 mg trastuzumab-dkst).
Use appropriate aseptic technique when performing the following reconstitution steps:
-
Using a sterile syringe, slowly inject the 7.4 mL of SWFI (not supplied) into the vial containing the lyophilized powder of Ogivri, which has a cake-like appearance. The stream of diluent should be directed into the cake. The reconstituted vial yields a solution for single-dose use, containing 21 mg/mL trastuzumab-dkst.
-
Swirl the vial gently to aid reconstitution. DO NOT SHAKE.
-
Slight foaming of the product may be present upon reconstitution. Allow the vial to stand undisturbed for approximately 5 minutes.
-
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Inspect visually for particulates and discoloration. The solution should be free of visible particulates, clear to slightly opalescent and colorless to pale yellow.
-
Use the Ogivri solution immediately following reconstitution with SWFI, as it contains no preservative and is intended for single-dose only. If not used immediately, store the reconstituted Ogivri solution for up to 24 hours at 2° to 8°C (36° to 46°F); discard any unused Ogivri after 24 hours. Do not freeze.
Dilution:
Determine the dose (mg) of Ogivri [see Dosage and Administration (2.1)].
-
Calculate the volume of the 21 mg/mL reconstituted Ogivri solution needed
-
Withdraw this amount from the vial and add it to an infusion bag containing 250 mL of 0.9% Sodium Chloride Injection, USP. DO NOT USE DEXTROSE (5%) SOLUTION.
-
Gently invert the bag to mix the solution.
-
The solution of Ogivri for infusion diluted in polyvinylchloride or polyethylene bags containing 0.9% Sodium Chloride Injection, USP, should be stored at 2° to 8°C (36° to 46°F) for no more than 24 hours prior to use. Discard after 24 hours. This storage time is
additional to the time allowed for the reconstituted vials. Do not freeze.
-
-
3 DOSAGE FORMS AND STRENGTHS
- •
- For injection: 150 mg of Ogivri as an off-white to pale yellow lyophilized powder in a single-dose vial.
- •
- For injection: 420 mg of Ogivri as an off-white to pale yellow lyophilized powder in a multiple-dose vial supplied with a separate vial containing 20 mL of Bacteriostatic Water for Injection (BWFI), to be used as a diluent.
- •
- For injection: 420 mg of Ogivri as an off-white to pale yellow lyophilized powder in a multiple-dose vial. No diluent is provided.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Cardiomyopathy
Trastuzumab products can cause left ventricular cardiac dysfunction, arrhythmias, hypertension, disabling cardiac failure, cardiomyopathy, and cardiac death [see Boxed Warning: Cardiomyopathy]. Trastuzumab products can also cause asymptomatic decline in left ventricular ejection fraction (LVEF).
There is a 4 to 6 fold increase in the incidence of symptomatic myocardial dysfunction among patients receiving trastuzumab products as a single agent or in combination therapy compared with those not receiving trastuzumab products. The highest absolute incidence occurs when a trastuzumab product is administered with an anthracycline.
Withhold Ogivri for ≥ 16% absolute decrease in LVEF from pre-treatment values or an LVEF value below institutional limits of normal and ≥ 10% absolute decrease in LVEF from pretreatment values [see Dosage and Administration (2.5)]. The safety of continuation or resumption of Ogivri in patients with trastuzumab product-induced left ventricular cardiac dysfunction has not been studied.
Patients who receive anthracycline after stopping Ogivri may also be at increased risk of cardiac dysfunction [see Drug Interactions (7) and Clinical Pharmacology (12.3)].
Cardiac Monitoring
Conduct thorough cardiac assessment, including history, physical examination, and determination of LVEF by echocardiogram or MUGA scan. The following schedule is recommended:
-
Baseline LVEF measurement immediately prior to initiation of Ogivri
-
LVEF measurements every 3 months during and upon completion of Ogivri
-
Repeat LVEF measurement at 4 week intervals if Ogivri is withheld for significant left ventricular cardiac dysfunction [see Dosage and Administration (2.4)]
-
LVEF measurements every 6 months for at least 2 years following completion of Ogivri as a component of adjuvant therapy.
In NSABP B31, 15% (158/1031) of patients discontinued trastuzumab due to clinical evidence of myocardial dysfunction or significant decline in LVEF after a median follow-up duration of 8.7 years in the AC-TH (anthracycline, cyclophosphamide, paclitaxel, and trastuzumab) arm. In HERA (one-year trastuzumab treatment), the number of patients who discontinued trastuzumab due to cardiac toxicity at 12.6 months median duration of follow-up was 2.6% (44/1678). In BCIRG006, a total of 2.9% (31/1056) of patients in the TCH (docetaxel, carboplatin, trastuzumab) arm (1.5% during the chemotherapy phase and 1.4% during the monotherapy phase) and 5.7% (61/1068) of patients in the AC-TH arm (1.5% during the chemotherapy phase and 4.2% during the monotherapy phase) discontinued trastuzumab due to cardiac toxicity.
Among 64 patients receiving adjuvant chemotherapy (NSABP B31 and NCCTG N9831) who developed congestive heart failure, one patient died of cardiomyopathy, one patient died suddenly without documented etiology and 33 patients were receiving cardiac medication at last follow-up. Approximately 24% of the surviving patients had recovery to a normal LVEF (defined as ≥ 50%) and no symptoms on continuing medical management at the time of last follow-up. Incidence of congestive heart failure is presented in Table 1. The safety of continuation or resumption of Ogivri in patients with trastuzumab -induced left ventricular cardiac dysfunction has not been studied.
Table 1: Incidence of Congestive Heart Failure in Adjuvant Breast Cancer Studies
Incidence of Congestive Heart Failure
% (n)
Study Regimen Trastuzumab Control NSABP
B31 & NCCTG N9831a
ACb → Paclitaxel + Trastuzumab 3.2% (64/2000)c 1.3% (21/1655) HERAd Chemotherapy →Trastuzumab 2% (30/1678) 0.3% (5/1708) BCIRG0
06
ACb → Docetaxel + Trastuzumab 2% (20/1068) 0.3% (3/1050) BCIRG0
06
Docetaxel + Carbo + Trastuzumab 0.4% (4/1056) 0.3% (3/1050) a Median follow-up duration for NSABP B31 & NCCTG N9831 combined was 8.3 years in the AC → paclitaxel + trastuzumab arm.
b Anthracycline (doxorubicin) and cyclophosphamide.
c Includes 1 patient with fatal cardiomyopathy and 1 patient with sudden death without documented etiology.
d Includes NYHA II-IV and cardiac death at 12.6 months median duration of follow-up in the one-year trastuzumab arm.
In HERA (one-year trastuzumab treatment), at a median follow-up duration of 8 years, the incidence of severe CHF (NYHA III & IV) was 0.8%, and the rate of mild symptomatic and asymptomatic left ventricular dysfunction was 4.6%.
Table 2:Incidence of Cardiac Dysfunctiona in Metastatic Breast Cancer Studies
Incidence NYHA I−IV NYHA III−IV Study Event Trastuzumab Control Trastuzumab Control H0648g (AC)b Cardiac Dysfunction 28% 7% 19% 3% H0648g
(paclitaxel)
Cardiac Dysfunction 11% 1% 4% 1% H0649g Cardiac Dysfunctionc 7% N/A 5% N/A a Congestive heart failure or significant asymptomatic decrease in LVEF.
b Anthracycline (doxorubicin or epirubicin) and cyclophosphamide.
c Includes 1 patient with fatal cardiomyopathy.
In BCIRG006, the incidence of NCI-CTC Grade 3/4 cardiac ischemia/infarction was higher in the trastuzumab containing regimens (AC-TH: 0.3% (3/1068) and TCH: 0.2% (2/1056)) as compared to none in AC-T.
5.2 Infusion Reactions
Infusion reactions consist of a symptom complex characterized by fever and chills, and on occasion included nausea, vomiting, pain (in some cases at tumor sites), headache, dizziness, dyspnea, hypotension, rash, and asthenia [see Adverse Reactions (6.1)].
In post-marketing reports, serious and fatal infusion reactions have been reported. Severe reactions, which include bronchospasm, anaphylaxis, angioedema, hypoxia, and severe hypotension, were usually reported during or immediately following the initial infusion. However, the onset and clinical course were variable, including progressive worsening, initial improvement followed by clinical deterioration, or delayed post-infusion events with rapid clinical deterioration. For fatal events, death occurred within hours to days following a serious infusion reaction.
Interrupt Ogivri infusion in all patients experiencing dyspnea, clinically significant hypotension, and intervention of medical therapy administered (which may include epinephrine, corticosteroids, diphenhydramine, bronchodilators, and oxygen). Patients should be evaluated and carefully monitored until complete resolution of signs and symptoms. Permanent discontinuation should be strongly considered in all patients with severe infusion reactions.
There are no data regarding the most appropriate method of identification of patients who may safely be retreated with trastuzumab products after experiencing a severe infusion reaction. Prior to resumption of trastuzumab infusion, the majority of patients who experienced a severe infusion reaction were pre-medicated with antihistamines and/or corticosteroids. While some patients tolerated trastuzumab infusions, others had recurrent severe infusion reactions despite pre-medications.
5.3 Embryo-Fetal Toxicity
Trastuzumab products can cause fetal harm when administered to a pregnant woman. In post-marketing reports, use of trastuzumab during pregnancy resulted in cases of oligohydramnios and oligohydramnios sequence manifesting as pulmonary hypoplasia, skeletal abnormalities, and neonatal death.
Verify the pregnancy status of females of reproductive potential prior to the initiation of Ogivri. Advise pregnant women and females of reproductive potential that exposure to Ogivri during pregnancy or within 7 months prior to conception can result in fetal harm. Advise females of reproductive potential to use effective contraception during treatment and for 7 months following the last dose of Ogivri [see Use in Specific Populations (8.1, 8.3) and Clinical Pharmacology (12.3)].
5.4 Pulmonary Toxicity
Trastuzumab product use can result in serious and fatal pulmonary toxicity. Pulmonary toxicity includes dyspnea, interstitial pneumonitis, pulmonary infiltrates, pleural effusions, non-cardiogenic pulmonary edema, pulmonary insufficiency and hypoxia, acute respiratory distress syndrome, and pulmonary fibrosis. Such events can occur as sequelae of infusion reactions [see Warnings and Precautions (5.2)]. Patients with symptomatic intrinsic lung disease or with extensive tumor involvement of the lungs, resulting in dyspnea at rest, appear to have more severe toxicity.
5.5 Exacerbation of Chemotherapy-Induced Neutropenia
In randomized, controlled clinical trials, the per-patient incidences of NCI-CTC Grade 3 to 4 neutropenia and of febrile neutropenia were higher in patients receiving trastuzumab in combination with myelosuppressive chemotherapy as compared to those who received chemotherapy alone. The incidence of septic death was similar among patients who received trastuzumab and those who did not [see Adverse Reactions (6.1)].
-
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the label:
-
Cardiomyopathy [see Warnings and Precautions (5.1)]
-
Infusion Reactions [see Warnings and Precautions (5.2)]
-
Embryo-Fetal Toxicity [see Warnings and Precautions (5.3)]
-
Pulmonary Toxicity [see Warnings and Precautions (5.4)]
-
Exacerbation of Chemotherapy-induced Neutropenia [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most common adverse reactions in patients receiving trastuzumab products in the adjuvant and metastatic breast cancer setting are fever, nausea, vomiting, infusion reactions, diarrhea, infections, increased cough, headache, fatigue, dyspnea, rash, neutropenia, anemia, and myalgia. Adverse reactions requiring interruption or discontinuation of trastuzumab product treatment include CHF, significant decline in left ventricular cardiac function, severe infusion reactions, and pulmonary toxicity [see Dosage and Administration (2.5)].
In the metastatic gastric cancer setting, the most common adverse reactions (≥ 10%) that were increased (≥ 5% difference) in patients receiving trastuzumab as compared to patients receiving chemotherapy alone were neutropenia, diarrhea, fatigue, anemia, stomatitis, weight loss, upper respiratory tract infections, fever, thrombocytopenia, mucosal inflammation, nasopharyngitis, and dysgeusia.
The most common adverse reactions which resulted in discontinuation of trastuzumab treatment in the absence of disease progression were infection, diarrhea, and febrile neutropenia.
Adjuvant Breast Cancer Studies
The information below reflect exposure to one-year trastuzumab therapy across three randomized, open-label studies, Studies 1, 2, and 3, with (n = 3678) or without (n = 3363) trastuzumab in the adjuvant treatment of breast cancer.
HERA
Table 3 reflects exposure to trastuzumab in 1678 patients; the median treatment duration was 51 weeks and median number of infusions was 18 [see Clinical Studies (14.1)].
Table 3: Adverse Reactions (>1%) in HERA (All Grades)a
Adverse Reactions Trastuzumab
(n = 1678)
%
Observation
(n = 1708)
%
Nervous System Headache 10 3 Paresthesia 2 0.6 Musculoskeletal Arthralgia 8 6 Back pain 5 3 Myalgia 4 1 Bone pain 3 2 Muscle spasm 3 0.2 Infections Nasopharyngitis 8 3 Urinary tract infection 3 0.8 Gastrointestinal Diarrhea 7 1 Nausea 6 1 Vomiting 3.5 0.6 Constipation 2 1 Dyspepsia 2 0.5 Upper abdominal pain 2 1 General Pyrexia 6 0.4 Peripheral edema 5 2 Chills 5 0 Asthenia 4.5 2 Influenza-like illness 2 0.2 Respiratory Thoracic Mediastinal Cough 5 2 Influenza 4 0.5 Dyspnea 3 2 URI 3 1 Rhinitis 2 0.4 Pharyngolaryngeal pain 2 0.5 Sinusitis 2 0.3 Cardiac Hypertension 4 2 Dizziness 4 2 Ejection fraction decreased 3.5 0.6 Palpitations 3 0.7 Cardiac arrhythmiasb 3 1 Cardiac failure (congestive) 2 0.3 Skin & Subcutaneous Tissue Rash 4 0.6 Nail disorders 2 0 Pruritus 2 0.6 aThe incidence of Grade 3 or higher adverse reactions was < 1% in both arms for each listed term.
b Higher level grouping term.
Clinically relevant adverse reactions in < 1% of patients who received trastuzumab in HERA included hypersensitivity (0.6%), cardiac failure (0.5%), cardiac disorder (0.3%), interstitial pneumonitis (0.2%), pulmonary hypertension (0.2%), ventricular disorder (0.2%), autoimmune thyroiditis (0.3%), and sudden death (0.06%).
Adjuvant Treatment of Breast Cancer with Trastuzumab Beyond One Year
Extending adjuvant treatment beyond one year is not recommended [see Dosage and Administration (2.2)]. In HERA, a comparison of trastuzumab administered once every 3 weeks for two years versus one year was performed. The rate of asymptomatic cardiac dysfunction was increased in the 2-year trastuzumab compared to the 1-year trastuzumab treatment arm (8.1% versus 4.6%, respectively). More patients experienced at least one adverse reaction of Grade 3 or higher in the 2-year trastuzumab treatment arm (20.4%) compared with the one-year trastuzumab treatment arm (16.3%).
NSABP B31 and NCCTG N9831
The safety data from NSABP B31 and NCCTG N9831 were obtained from 3655 patients, of whom 2000 received trastuzumab; the median treatment duration was 51 weeks [see Clinical Studies (14.1)].
In NSABP B31, only Grade 3 to 5 adverse events, treatment-related Grade 2 events, and Grade 2 to 5 dyspnea were collected during and for up to 3 months following protocol-specified treatment. The following non-cardiac adverse reactions of Grade 2 to 5 occurred at an incidence of at least 2% greater among patients receiving trastuzumab plus chemotherapy as compared to chemotherapy alone: fatigue (29.5% vs. 22.4%), infection (24.0% vs. 12.8%), hot flashes (17.1% vs. 15%), anemia (12.3% vs. 6.7%), dyspnea (11.8% vs. 4.6%), rash/desquamation (10.9% vs. 7.6%), leukopenia (10.5% vs. 8.4%), neutropenia (6.4% vs. 4.3%), headache (6.2% vs. 3.8%), pain (5.5% vs. 3.0%), edema (4.7% vs. 2.7%) and insomnia (4.3% vs. 1.5%). The majority of these events were Grade 2 in severity.
In NCCTG N9831, data collection was limited to the following investigator-attributed treatment-related adverse reactions: NCI-CTC Grade 4 and 5 hematologic toxicities, Grade 3 to 5 non-hematologic toxicities, selected Grade 2 to 5 toxicities associated with taxanes (myalgia, arthralgias, nail changes, motor neuropathy, sensory neuropathy) and Grade 1 to 5 cardiac toxicities occurring during chemotherapy and/or trastuzumab treatment. The following non-cardiac adverse reactions of Grade 2 to 5 occurred at an incidence of at least 2% greater among patients receiving trastuzumab plus chemotherapy as compared to chemotherapy alone: arthralgia (12.2% vs. 9.1%), nail changes (11.5% vs.6.8%), dyspnea (2.4% vs. 0.2%), and diarrhea (2.2% vs. 0%). The majority of these events were Grade 2 in severity.
BCIRG006
Safety data from BCIRG006reflect exposure to trastuzumab as part of an adjuvant treatment regiimen from 2124 patients receiving at least one dose of study treatment [AC-TH: n = 1068; TCH: n = 1056]. The overall median treatment duration was 54 weeks in both the AC-TH and TCH arms. The median number of infusions was 26 in the AC-TH arm and 30 in the TCH arm, including weekly infusions during the chemotherapy phase and once every three week dosing in the monotherapy period [see Clinical Studies (14.1)]. In BCIRG006, the toxicity profile was similar to that reported in Studies NSABP B31, NCCTG N9831, and HERA with the exception of a low incidence of CHF in the TCH arm.
Metastatic Breast Cancer Studies
The safety of trastuzumab was evaluated in one randomized, open-label study (H0648g) of chemotherapy with (n = 235) or without (n = 234) intravenous trastuzumab in patients with metastatic breast cancer and in one single-arm study (H0649g); in patients with metastatic breast cancer (n=222) [see Clinical Studies (14.1)]. Patients received 4 mg/kg initial dose of trastuzumab followed by 2 mg/kg weekly. In H0648g, 58% of patients received trastuzumab for ≥ 6 months and 9% received trastuzumab ≥ 12 months, respectively. In H0649g, 31% of patients received trastuzumab for ≥ 6 months and 16% received trastuzumab for ≥ 12 months, respectively.
Table 4 shows the adverse reactions (≥ 5%) in patients from H0648g and H0649g.
Table 4: Adverse Reactions³ (5%) in the Trastuzumab Arms in H0648g and H0649g
Trastuzumaba n = 352
%
Trastuzumab +
Paclitaxel
n = 91
%
Paclitaxel n = 95
%
Trastuzumab +
ACb
n = 143
%
ACb
n = 135 %
General Pain 47 61 62 57 42 Asthenia 42 62 57 54 55 Fever 36 49 23 56 34 Chills 32 41 4 35 11 Headache 26 36 28 44 31 Abdominal pain 22 34 22 23 18 Back pain 22 34 30 27 15 Infection 20 47 27 47 31 Flu syndrome 10 12 5 12 6 Accidental injury 6 13 3 9 4 Allergic reaction 3 8 2 4 2 Gastrointestinal Nausea 33 51 9 76 77 Diarrhea 25 45 29 45 26 Vomiting 23 37 28 53 49 Anorexia 14 24 16 31 26 Nausea and vomiting 8 14 11 18 9 Respiratory Cough increased 26 41 22 43 29 Dyspnea 22 27 26 42 25 Rhinitis 14 22 5 22 16 Pharyngitis 12 22 14 30 18 Sinusitis 9 21 7 13 6 Skin Rash 18 38 18 27 17 Herpes simplex 2 12 3 7 9 Acne 2 11 3 3 < 1 Nervous Insomnia 14 25 13 29 15 Dizziness 13 22 24 24 18 Paresthesia 9 48 39 17 11 Depression 6 12 13 20 12 Peripheral neuritis 2 23 16 2 2 Neuropathy 1 13 5 4 4 Metabolic Peripheral edema 10 22 20 20 17 Edema 8 10 8 11 5 Cardiovascular Congestive heart failure 7 11 1 28 7 Tachycardia 5 12 4 10 5 Table 4: Adverse Reactions (> 5%) in the Trastuzumab Arms in H0648g and H0649g (continued)
Trastuzumaba
n = 352
%
Trastuzumab +
Paclitaxel
n = 91
%
Paclitaxel
n = 95
%
Trastuzumab +
ACb
n = 143
%
ACb
n =135
%
Musculoskeletal Bone pain 7 24 18 7 7 Arthralgia 6 37 21 8 9 Urogenital Urinary tract infection 5 18 14 13 7 Blood and Lymphatic Anemia 4 14 9 36 26 Leukopenia 3 24 17 52 34 a Data for trastuzumab single agent were from 4 studies, including 213 patients from H0649g.
b Anthracycline (doxorubicin or epirubicin) and cyclophosphamide.
Metastatic Gastric Cancer
The safety of trastuzumab was evaluated in patients with previously untreated for metastatic gastric or gastroesophageal junction adenocarcinoma in an open label, multi-center trial (ToGA) [see Clinical Studies (14.3)]. Patients were randomized (1:1) to receive trastuzumab in combination with cisplatin and a fluoropyrimidine (FC+H) (n=294) or chemotherapy alone (FC) (n=290) Patients in the trastuzumab plus chemotherapy arm received trastuzumab 8 mg/kg administered on Day 1 (prior to chemotherapy) followed by 6 mg/kg every 21 days until disease progression. Cisplatin was administered at 80 mg/m2 on Day 1 and the fluoropyrimidine was administered as either capecitabine 1000 mg/m2 orally twice a day on Days 1 to 14 or 5-fluorouracil 800 mg/m2/day as a continuous intravenous infusion Days 1 through 5. Chemotherapy was administered for six 21-day cycles. Median duration of traztuzumab treatment was 21 weeks and the median number of trastuzumab infusions administered was eight.
Table 5: Adverse Reactions (All Grades > 5% or Grade 3-4 > 1% between Arms) in ToGA
Adverse Reactions Trastuzumab + FC
(N = 294) %
FC
(N = 290)
%
All Grades Grades 3 to 4 All Grades Grades 3 to 4 Investigations Neutropenia 78 34 73 29 Hypokalemia 28 10 24 6 Anemia 28 12 21 10 Thrombocytopenia 16 5 11 3 Blood and Lymphatic System Disorders Febrile Neutropenia - 5 - 3 Gastrointestinal Disorders Diarrhea 37 9 28 4 Stomatitis 24 1 15 2 Dysphagia 6 2 3 < 1 General Fatigue 35 4 28 2 Fever 18 1 12 0 Mucosal Inflammation 13 2 6 1 Chills 8 < 1 0 0 Metabolism and Nutrition Disorders Weight Decrease 23 2 14 2 Infections and Infestations Upper Respiratory Tract Infections 19 0 10 0 Nasopharyngitis 13 0 6 0 Renal and Urinary Disorders Renal Failure and Impairment 18 3 15 2 Nervous System Disorders Dysgeusia 10 0 5 0 The following subsections provide additional detail regarding adverse reactions observed in clinical trials of adjuvant breast cancer, metastatic breast cancer, metastatic gastric cancer, or post-marketing experience.
Cardiomyopathy
Serial measurement of cardiac function (LVEF) was obtained in clinical trials in the adjuvant treatment of breast cancer. In HERA, the median duration of follow-up was 12.6 months (12.4 months in the observation arm; 12.6 months in the 1-year trastuzumab arm); and in NSABP B31 and NCCTG N9831, 7.9 years in the AC-T arm, 8.3 years in the AC-TH arm. Following initiation of trastuzumab therapy, the incidence of new-onset dose-limiting myocardial dysfunction was higher among patients receiving trastuzumab and paclitaxel as compared to those receiving paclitaxel alone in NSABP B31 and NCCTG N9831, and in patients receiving one-year trastuzumab monotherapy compared to observation in HERA (see Table 6, Figures 1 and 2). The incidence of new-onset cardiac dysfunction, as measured by LVEF, remained similar when compared to the analysis performed at a median follow-up of 2.0 years in the AC-TH arm. This analysis also showed evidence of reversibility of left ventricular dysfunction, with 64.5% of patients who experienced symptomatic CHF in the AC-TH group being asymptomatic at latest follow-up, and 90.3% having full or partial LVEF recovery.
Table 6: Myocardial Dysfunction (by LVEF) Studies 1, 2, 3 and 4a
Study and Arm LVEF <50%
and Decrease from Baseline
LVEF Decrease LVEF
< 50%
≥ 10%
decrease
≥ 16%
decrease
< 20% and ≥ 10% ≥ 20% Studies 1 & 2b,c AC → TH
(n = 1856)
23.1%
(428)
18.5%
(344)
11.2%
(208)
37.9%
(703)
8.9%
(166)
AC → T
(n = 1170)
11.7%
(137)
7.0%
(82)
3.0%
(35)
22.1%
(259)
3.4%
(40)
HERAd Trastuzumab
(n = 1678)
8.6%
(144)
7.0%
(118)
3.8%
(64)
22.4%
(376)
3.5%
(59)
Observation
(n = 1708)
2.7%
(46)
2.0%
(35)
1.2%
(20)
11.9%
(204)
1.2%
(21)
BCIRG006e TCH
(n = 1056)
8.5%
(90)
5.9%
(62)
3.3%
(35)
34.5%
(364)
6.3%
(67)
AC → TH
(n = 1068)
17%
(182)
13.3%
(142)
9.8%
(105)
44.3%
(473)
13.2%
(141)
AC → T
(n = 1050)
9.5%
(100)
6.6%
(69)
3.3%
(35)
34%
(357)
5.5%
(58)
a For Studies 1, 2 and 3, events are counted from the beginning of trastuzumab treatment. For BCIRG006, events are counted from the date of randomization.
b NSABP B31 and NCCTG N9831 regimens: doxorubicin and cyclophosphamide followed by paclitaxel (AC → T) or paclitaxel plus trastuzumab (AC → TH).
c Median duration of follow-up for NSABP B31 and NCCTG N9831 combined was 8.3 years in the AC → TH arm.
d Median follow-up duration of 12.6 months in the one-year trastuzumab treatment arm.
e BCIRG006regimens: doxorubicin and cyclophosphamide followed by docetaxel (AC → T) or docetaxel plus trastuzumab (AC → TH); docetaxel and carboplatin plus trastuzumab (TCH).
Figure 1
NSABP B31 and NCCTG N9831: Cumulative Incidence of Time to First LVEF
Decline of ≥ 10 Percentage Points from Baseline and toBelow 50% with Death as a Competing Risk Event
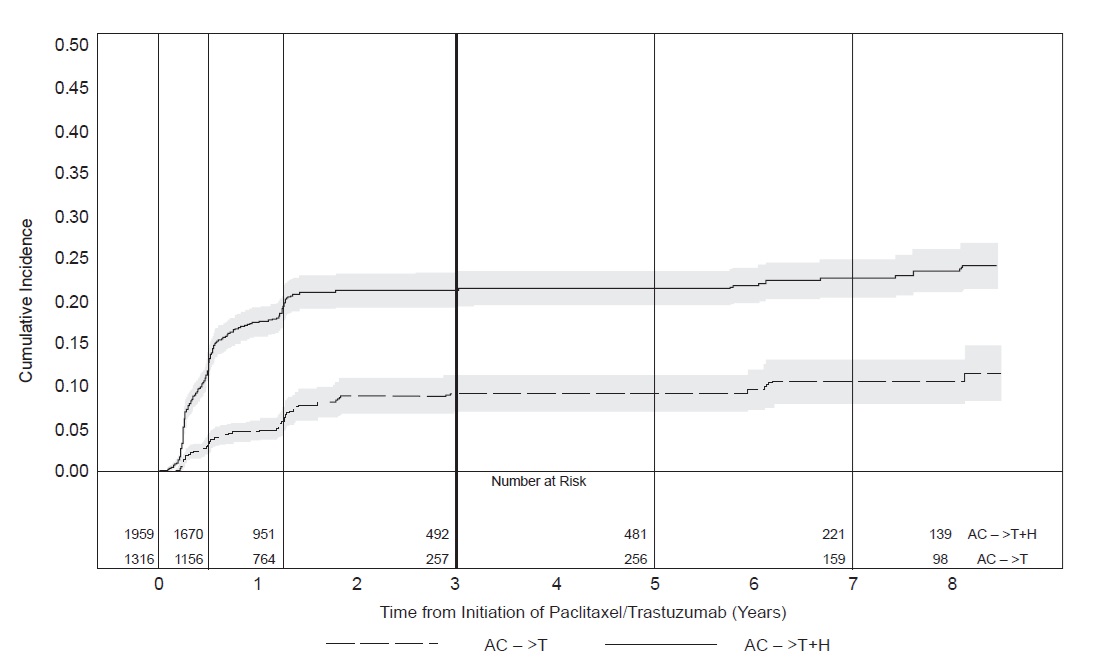
Time 0 is initiation of paclitaxel or trastuzumab + paclitaxel therapy.
Figure 2
HERA: Cumulative Incidence of Time to First LVEF
Decline of ≥ 10 Percentage Points from Baseline and to Below 50% with Death as a Competing Risk Event
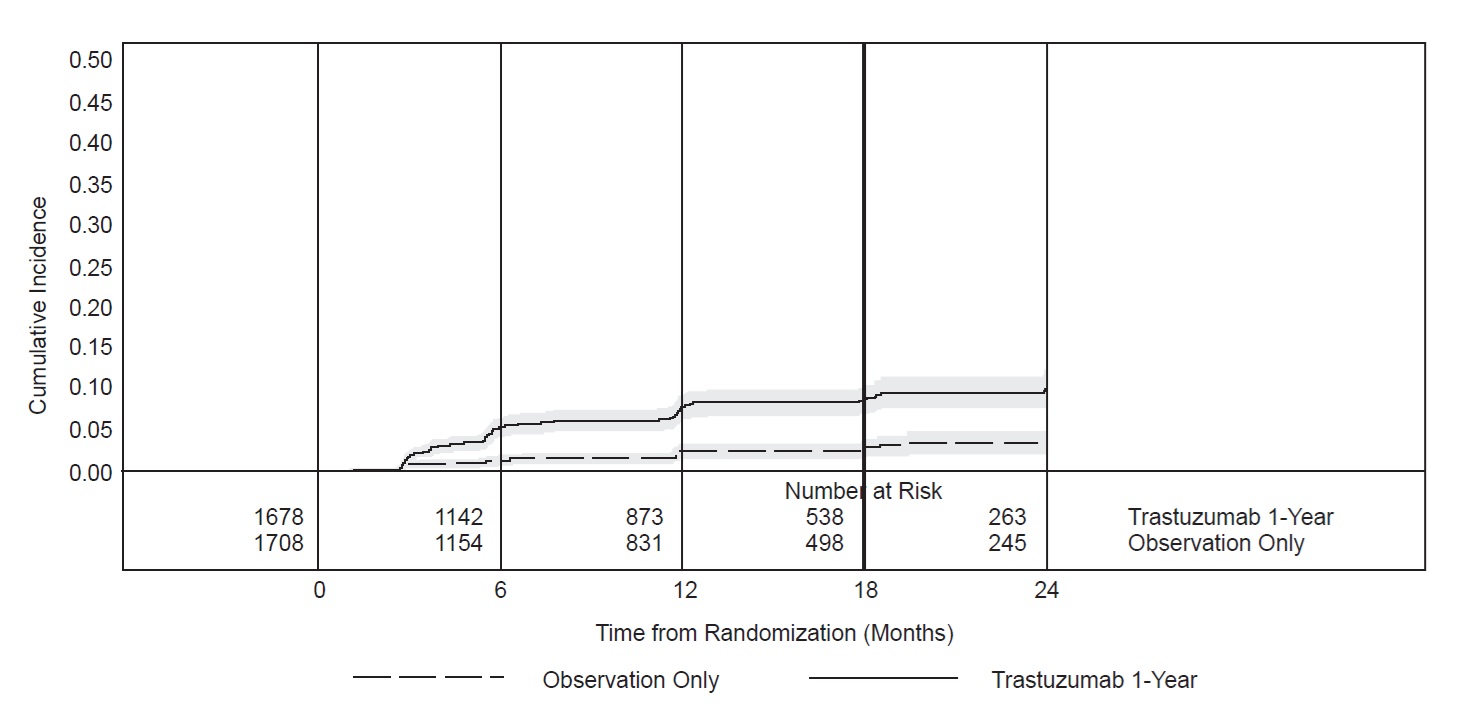
Time 0 is the date of randomization.
Figure 3
BCIRG006: Cumulative Incidence of Time to First LVEF
Decline of ≥ 10 Percentage Points from Baseline and to Below 50% with Death as a Competing Risk Event
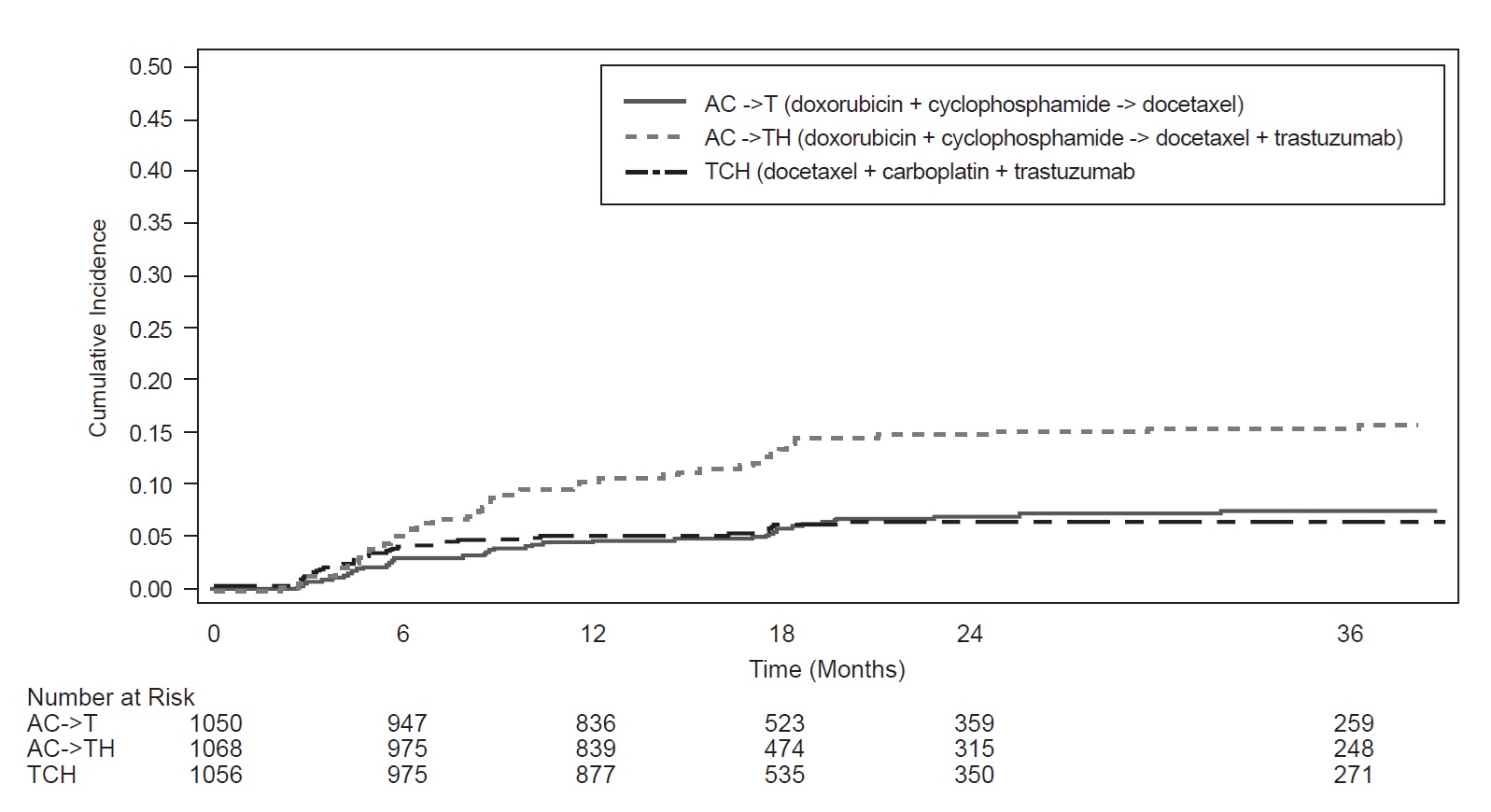
Time 0 is the date of randomization.
The incidence of treatment emergent congestive heart failure among patients in the metastatic breast cancer trials was classified for severity using the New York Heart Association classification system (I−IV, where IV is the most severe level of cardiac failure) (see Table 2). In the metastatic breast cancer trials, the probability of cardiac dysfunction was highest in patients who received trastuzumab concurrently with anthracyclines.
In ToGA, 5.0% of patients in the trastuzumab plus chemotherapy arm compared to 1.1% of patients in the chemotherapy alone arm had LVEF value below 50% with a ≥ 10% absolute decrease in LVEF from pretreatment values.
Infusion Reactions
During the first infusion with trastuzumab, the symptoms most commonly reported were chills and fever, occurring in approximately 40% of patients in clinical trials. Symptoms were treated with acetaminophen, diphenhydramine, and meperidine (with or without reduction in the rate of trastuzumab infusion); permanent discontinuation of trastuzumab for infusion reactions was required in < 1% of patients. Other signs and/or symptoms may include nausea, vomiting, pain (in some cases at tumor sites), rigors, headache, dizziness, dyspnea, hypotension, elevated blood pressure, rash, and asthenia. Infusion reactions occurred in 21% and 35% of patients, and were severe in 1.4% and 9% of patients, on second or subsequent trastuzumab infusions administered as monotherapy or in combination with chemotherapy, respectively. In the post-marketing setting, severe infusion reactions, including hypersensitivity, anaphylaxis, and angioedema have been reported.
Anemia
In randomized controlled clinical trials, the overall incidence of anemia (30% vs. 21% [H0648g]), of selected NCI-CTC Grade 2 to 5 anemia (12.3% vs. 6.7% [NSABP B31]), and of anemia requiring transfusions (0.1% vs. 0 patients [NCCTG N9831]) were increased in patients receiving trastuzumab and chemotherapy compared with those receiving chemotherapy alone. Following the administration of trastuzumab as a single agent (H0649g), the incidence of NCI-CTC Grade 3 anemia was < 1%. In ToGA (metastatic gastric cancer), on the trastuzumab containing arm as compared to the chemotherapy alone arm, the overall incidence of anemia was 28% compared to 21% and of NCICTC Grade 3/4 anemia was 12.2% compared to 10.3%.
Neutropenia
In randomized controlled clinical trials in the adjuvant setting, the incidence of selected NCI-CTC Grade 4 to 5 neutropenia (1.7% vs. 0.8% [NCCTG N9831]) and of selected Grade 2 to 5 neutropenia (6.4% vs. 4.3% [NSABP B31]) were increased in patients receiving trastuzumab and chemotherapy compared with those receiving chemotherapy alone. In a randomized, controlled trial in patients with metastatic breast cancer, the incidences of NCI-CTC Grade 3/4 neutropenia (32% vs. 22%) and of febrile neutropenia (23% vs. 17%) were also increased in patients randomized to trastuzumab in combination with myelosuppressive chemotherapy as compared to chemotherapy alone. In ToGA (metastatic gastric cancer) on the trastuzumab containing arm as compared to the chemotherapy alone arm, the incidence of NCI-CTC Grade 3/4 neutropenia was 36.8% compared to 28.9%; febrile neutropenia 5.1% compared to 2.8%.
Infection
The overall incidences of infection (46% vs. 30% [H0648g]), of selected NCI-CTC Grade 2 to 5 infection/febrile neutropenia (24.3% vs. 13.4% [NSABP B31]) and of selected Grade 3 to 5 infection/febrile neutropenia (2.9% vs. 1.4%) [NCCTG N9831]) were higher in patients receiving trastuzumab and chemotherapy compared with those receiving chemotherapy alone. The most common site of infections in the adjuvant setting involved the upper respiratory tract, skin, and urinary tract.
In BCIRG006, the overall incidence of infection was higher with the addition of trastuzumab to AC-T but not to TCH [44% (AC-TH), 37% (TCH), 38% (AC-T)]. The incidences of NCI-CTC Grade 3 to 4 infection were similar [25% (AC-TH), 21% (TCH), 23% (AC-T)] across the three arms.
In a randomized, controlled trial in treatment of metastatic breast cancer, the reported incidence of febrile neutropenia was higher (23% vs. 17%) in patients receiving trastuzumab in combination with myelosuppressive chemotherapy as compared to chemotherapy alone.
Pulmonary Toxicity
Adjuvant Breast Cancer
Among women receiving adjuvant therapy for breast cancer, the incidence of selected NCI-CTC Grade 2 to 5 pulmonary toxicity (14.3% vs. 5.4% [NSABP B31]) and of selected NCI-CTC Grade 3 to 5 pulmonary toxicity and spontaneous reported Grade 2 dyspnea (3.4 % vs. 0.9% [NCCTG N9831]) was higher in patients receiving trastuzumab and chemotherapy compared with chemotherapy alone.
The most common pulmonary toxicity was dyspnea (NCI-CTC Grade 2 to 5: 11.8% vs. 4.6% [NSABP B31]; NCI-CTC Grade 2 to 5: 2.4% vs. 0.2% [NCCTG N9831]).
Pneumonitis/pulmonary infiltrates occurred in 0.7% of patients receiving trastuzumab compared with 0.3% of those receiving chemotherapy alone. Fatal respiratory failure occurred in 3 patients receiving trastuzumab, one as a component of multi-organ system failure, as compared to 1 patient receiving chemotherapy alone.
In HERA, there were 4 cases of interstitial pneumonitis in the one-year trastuzumab treatment arm compared to none in the observation arm at a median follow-up duration of 12.6 months.
Metastatic Breast Cancer
Among women receiving trastuzumab for treatment of metastatic breast cancer, the incidence of pulmonary toxicity was also increased. Pulmonary adverse events have been reported in the post-marketing experience as part of the symptom complex of infusion reactions. Pulmonary events include bronchospasm, hypoxia, dyspnea, pulmonary infiltrates, pleural effusions, non-cardiogenic pulmonary edema, and acute respiratory distress syndrome. For a detailed description, see Warnings and Precautions (5.4).
Thrombosis/Embolism
In 4 randomized, controlled clinical trials, the incidence of thrombotic adverse events was higher in patients receiving trastuzumab and chemotherapy compared to chemotherapy alone in three studies (2.6% vs. 1.5% [NSABP B31], 2.5% and 3.7% vs. 2.2% [BCIRG006] and 2.1% vs. 0% [H0648g]).
Diarrhea
Among women receiving adjuvant therapy for breast cancer, the incidence of NCI-CTC Grade 2 to 5 diarrhea (6.7% vs. 5.4% [NSABP B31]) and of NCI-CTC Grade 3 to 5 diarrhea (2.2% vs. 0% [NCCTG N9831]), and of Grade 1 to 4 diarrhea (7% vs. 1% [HERA; one-year trastuzumab treatment at 12.6 months median duration of follow-up]) were higher in patients receiving trastuzumab as compared to controls. In BCIRG006, the incidence of Grade 3 to 4 diarrhea was higher [5.7% AC-TH, 5.5% TCH vs. 3.0% AC-T] and of Grade 1 to 4 was higher [51% AC-TH, 63% TCH vs. 43% AC-T] among women receiving trastuzumab. Of patients receiving trastuzumab as a single agent for the treatment of metastatic breast cancer, 25% experienced diarrhea. An increased incidence of diarrhea was observed in patients receiving trastuzumab in combination with chemotherapy for treatment of metastatic breast cancer.
Renal Toxicity
In ToGA (metastatic gastric cancer) on the trastuzumab-containing arm as compared to the chemotherapy alone arm the incidence of renal impairment was 18% compared to 14.5%. Severe (Grade 3/4) renal failure was 2.7% on the trastuzumab-containing arm compared to 1.7% on the chemotherapy only arm. Treatment discontinuation for renal insufficiency/failure was 2% on the trastuzumab-containing arm and 0.3% on the chemotherapy only arm.
In the post-marketing setting, rare cases of nephrotic syndrome with pathologic evidence of glomerulopathy have been reported. The time to onset ranged from 4 months to approximately 18 months from initiation of trastuzumab therapy. Pathologic findings included membranous glomerulonephritis, focal glomerulosclerosis, and fibrillary glomerulonephritis. Complications included volume overload and congestive heart failure.
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post-approval use of trastuzumab. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
-
Infusion reaction [see Warnings and Precautions (5.2)]
-
Oligohydramnios or oligohydramnios sequence, including pulmonary hypoplasia, skeletal abnormalities, and neonatal death [see Warnings and Precautions (5.3)]
-
Glomerulopathy [see Adverse Reactions (6.1)]
-
Immune thrombocytopenia
-
Tumor lysis syndrome (TLS): Cases of possible TLS have been reported in patients treated with trastuzumab products. Patients with significant tumor burden (e.g. bulky metastases) may be at a higher risk. Patients could present with hyperuricemia, hyperphosphatemia, and acute renal failure which may represent possible TLS. Providers should consider additional monitoring and/or treatment as clinically indicated.
-
-
7 DRUG INTERACTIONS
Anthracyclines
Patients who receive anthracycline after stopping trastuzumab products may be at increased risk of cardiac dysfunction because of trastuzumab’s estimated long washout period [see Clinical Pharmacology (12.3)]. If possible, avoid anthracycline-based therapy for up to 7 months after stopping trastuzumab. If anthracyclines are used, the patient’s cardiac function should be monitored carefully.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Trastuzumab products can cause fetal harm when administered to a pregnant woman. In post-marketing reports, use of trastuzumab during pregnancy resulted in cases of oligohydramnios and of oligohydramnios sequence, manifesting as pulmonary hypoplasia, skeletal abnormalities, and neonatal death (see Data). Apprise the patient of the potential risks to a fetus. There are clinical considerations if a trastuzumab product is used in a pregnant woman or if a patient becomes pregnant within 7 months following the last dose of a trastuzumab product (see Clinical Considerations).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
In post-marketing reports, use of trastuzumab during pregnancy resulted in cases of oligohydramnios and of oligohydramnios sequence, manifesting in the fetus as pulmonary hypoplasia, skeletal abnormalities and neonatal death. These case reports described oligohydramnios in pregnant women who received trastuzumab either alone or in combination with chemotherapy. In some case reports, amniotic fluid index increased after trastuzumab was stopped. In one case, trastuzumab therapy resumed after amniotic index improved, and oligohydramnios recurred.
Animal Data
In studies where trastuzumab was administered to pregnant cynomolgus monkeys during the period of organogenesis at doses up to 25 mg/kg given twice weekly (up to 25 times the recommended weekly human dose of 2 mg/kg), trastuzumab crossed the placental barrier during the early (Gestation Days 20 to 50) and late (Gestation Days 120 to 150) phases of gestation. The resulting concentrations of trastuzumab in fetal serum and amniotic fluid were approximately 33% and 25%, respectively, of those present in the maternal serum but were not associated with adverse developmental effects.
8.2 Lactation
Risk Summary
There is no information regarding the presence of trastuzumab products in human milk, the effects on the breastfed infant, or the effects on milk production. Published data suggest human IgG is present in human milk but does not enter the neonatal and infant circulation in substantial amounts. Trastuzumab was present in the milk of lactating Cynomolgus monkeys but not associated with neonatal toxicity (see Data). Consider the developmental and health benefits of breastfeeding along with the mother’s clinical need for Ogivri treatment and any potential adverse effects on the breastfed child from Ogivri or from the underlying maternal condition. This consideration should also take into account the trastuzumab product wash out period of 7 months [see Clinical Pharmacology (12.3)].
Data
In lactating cynomolgus monkeys, trastuzumab was present in breast milk at about 0.3% of maternal serum concentrations after pre-(beginning Gestation Day 120) and post-partum (through Post-partum Day 28) doses of 25 mg/kg administered twice weekly (25 times the recommended weekly human dose of 2 mg/kg of trastuzumab products). Infant monkeys with detectable serum levels of trastuzumab did not exhibit any adverse effects on growth or development from birth to 1 month of age.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to the initiation of Ogivri.
Contraception
Females
Trastuzumab products can cause embryo-fetal harm when administered during pregnancy. Advise females of reproductive potential to use effective contraception during treatment with Ogivri and for 7 months following the last dose of Ogivri [see Use in Specific Populations (8.1) and Clinical Pharmacology (12.3)].
8.4 Pediatric Use
The safety and effectiveness of trastuzumab products in pediatric patients have not been established.
8.5 Geriatric Use
Trastuzumab has been administered to 386 patients who were 65 years of age or over (253 in the adjuvant treatment and 133 in metastatic breast cancer treatment settings). The risk of cardiac dysfunction was increased in geriatric patients as compared to younger patients in both those receiving treatment for metastatic disease in Studies H0648g and H0649g, or adjuvant therapy in Studies NSABP B31 and NCCTG N9831. Limitations in data collection and differences in study design of the 4 studies of trastuzumab in adjuvant treatment of breast cancer preclude a determination of whether the toxicity profile of trastuzumab in older patients is different from younger patients. The reported clinical experience is not adequate to determine whether the efficacy improvements (ORR, TTP, OS, DFS) of trastuzumab treatment in older patients is different from that observed in patients < 65 years of age for metastatic disease and adjuvant treatment.
In ToGA (metastatic gastric cancer), of the 294 patients treated with trastuzumab, 108 (37%) were 65 years of age or older, while 13 (4.4%) were 75 and over. No overall differences in safety or effectiveness were observed.
-
11 DESCRIPTION
Trastuzumab-dkst is a humanized IgG1 kappa monoclonal antibody that selectively binds with high affinity to the extracellular domain of the human epidermal growth factor receptor 2 protein, HER2. Trastuzumab-dkst is produced by recombinant DNA technology in a mammalian cell (Chinese Hamster Ovary) culture.
Ogivri (trastuzumab-dkst) for injection is a sterile, off-white to pale yellow, preservative-free lyophilized powder with a cake-like appearance, for injection, for intravenous administration.
Each multiple-dose vial of Ogivri delivers 420 mg trastuzumab-dkst, D-sorbitol (322.6 mg), L-Histidine (6.0 mg), Histidine hydrochloride monohydrate (9.4 mg) and Polyethylene glycol 3350/Macrogol 3350 (94.1 mg). Hydrochloric acid or sodium hydroxide may be used to adjust the pH of the formulation buffer. Reconstitution with 20 mL of the appropriate diluent (BWFI or SWFI) yields a solution containing 21 mg/mL trastuzumab-dkst that delivers 20 mL (420 mg trastuzumab-dkst), at a pH of approximately 6. If Ogivri is reconstituted with SWFI without preservative, the reconstituted solution is considered single-dose.
Each single-dose vial of Ogivri delivers 150 mg trastuzumab-dkst, D-sorbitol (115.2 mg), L-Histidine (2.16 mg), L-Histidine hydrochloride monohydrate (3.36 mg) and Polyethylene glycol 3350/Macrogol 3350 (33.6 mg). Hydrochloric acid or sodium hydroxide may be used to adjust the pH of the formulation buffer. Reconstitution with 7.4 mL of sterile water for injection (SWFI) yields a solution containing 21 mg/mL trastuzumab-dkst that delivers 7.15 mL (150 mg trastuzumab-dkst), at a pH of approximately 6.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The HER2 (or c-erbB2) proto-oncogene encodes a transmembrane receptor protein of 185 kDa, which is structurally related to the epidermal growth factor receptor. Trastuzumab products have been shown, in both in vitro assays and in animals, to inhibit the proliferation of human tumor cells that overexpress HER2.
Trastuzumab products are mediators of antibody-dependent cellular cytotoxicity (ADCC). In vitro, trastuzumab product-mediated ADCC has been shown to be preferentially exerted on HER2 overexpressing cancer cells compared with cancer cells that do not overexpress HER2.
12.2 Pharmacodynamics
Trastuzumab exposure-response relationships and the time course of pharmacodynamic responses are not fully characterized.
Cardiac Electrophysiology
The effects of trastuzumab on electrocardiographic (ECG) endpoints, including QTc interval duration, were evaluated in patients with HER2 positive solid tumors. Trastuzumab had no clinically relevant effect on the QTc interval duration and there was no apparent relationship between serum trastuzumab concentrations and change in QTcF interval duration in patients with HER2 positive solid tumors.
12.3 Pharmacokinetics
The pharmacokinetics of trastuzumab were evaluated in a pooled population pharmacokinetic (PK) model analysis of 1,582 subjects with primarily breast cancer and metastatic gastric cancer (MGC) receiving intravenous trastuzumab. Total trastuzumab clearance increases with decreasing concentrations due to parallel linear and non-linear elimination pathways.
Although the average trastuzumab exposure was higher following the first cycle in breast cancer patients receiving the once every three week schedule compared to the weekly schedule of trastuzumab, the average steady-state exposure was essentially the same at both dosages. The average trastuzumab exposure following the first cycle and at steady state as well as the time to steady state was higher in breast cancer patients compared to MGC patients at the same dosage; however, the reason for this exposure difference is unknown. Additional predicted trastuzumab exposure and PK parameters following the first trastuzumab cycle and at steady state exposure are described in Tables 7 and 8, respectively.
Population PK based simulations indicate that following discontinuation of trastuzumab, concentrations in at least 95% of breast cancer patients and MGC patients will decrease to approximately 3% of the population predicted steady-state trough serum concentration (approximately 97% washout) by 7 months [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1, 8.3)].
Table 7
Population Predicted Cycle 1 PK Exposures (Median with 5th to 95th Percentiles) in Breast Cancer
and MGC Patients
Schedule Primary tumor
type
N Cmin
(µg/mL)
Cmax
(µg/mL)
AUC0-21days
(µg.day/mL)
8 mg/kg +
6 mg/kg q3w
Breast cancer 1195 29.4
(5.8 to 59.5)
178
(117 to 291)
1373
(736 to 2245)
MGC 274 23.1
(6.1 to50.3)
132
(84.2 to 225)
1109
(588 to 1938)
4 mg/kg +
2 mg/kg qw
Breast cancer 1195 37.7
(12.3 to 70.9)
88.3
(58 to 144)
1066
(586 to 1754)
Table 8
Population Predicted Steady State PK Exposures (Median with 5th to 95th Percentiles) in Breast
Cancer and MGC Patients
Schedule Primary tumor type N Cmin,ssa
(µg/mL)
Cmax,ssb
(µg/mL)
AUCss,0-21days
(µg.day/mL)
Time to steady-state (week) Total CL range at steady-state (L/day) 8 mg/kg +
6 mg/kg
q3w
Breast
cancer
1195 47.4
(5 to 115)
179
(107 to 309)
1794
(673 to 3618)
12 0.173 to 0.283 MGC 274 32.9
(6.1 to 88.9)
131
(72.5 to 251)
1338
(557 to 2875)
9 0.189 to 0.337 4 mg/kg +
2 mg/kg qw
Breast
cancer
1195 66.1
(14.9 to 142)
109
(51.0 to 209)
1765
(647 to 3578)
12 0.201 to 0.244 a Steady-state trough serum concentration of trastuzumab
b Maximum steady-state serum concentration of trastuzumab
Specific Populations
Based on a population pharmacokinetic analysis, no clinically significant differences were observed in the pharmacokinetics of trastuzumab based on age (<65 (n = 1294); ≥65 (n = 288)), race (Asian (n = 264); non-Asian (n = 1324)) and renal impairment (mild (creatinine clearance [CLcr] 60 to 90 mL/min) (n = 636) or moderate (CLcr 30 to 60 mL/min) (n = 133)). The pharmacokinetics of trastuzumab products in patients with severe renal impairment, end-stage renal disease with or without hemodialysis, or hepatic impairment is unknown.
Drug Interaction Studies
There have been no formal drug interaction studies performed with trastuzumab products in humans. Clinically significant interactions between trastuzumab and concomitant medications used in clinical trials have not been observed.
Paclitaxel and doxorubicin: Concentrations of paclitaxel and doxorubicin and their major metabolites (i.e., 6-α hydroxyl-paclitaxel [POH], and doxorubicinol [DOL], respectively) were not altered in the presence of trastuzumab when used as combination therapy in clinical trials. Trastuzumab concentrations were not altered as part of this combination therapy.
Docetaxel and carboplatin: When trastuzumab was administered in combination with docetaxel or carboplatin, neither the plasma concentrations of docetaxel or carboplatin nor the plasma concentrations of trastuzumab were altered.
Cisplatin and capecitabine: In a drug interaction substudy conducted in patients in ToGA, the pharmacokinetics of cisplatin, capecitabine and their metabolites were not altered when administered in combination with trastuzumab.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of trastuzumab or of other trastuzumab products.
Among 903 women with metastatic breast cancer, human anti human antibody (HAHA) to trastuzumab was detected in one patient using an enzyme linked immunosorbent assay (ELISA). This patient did not experience an allergic reaction. Samples for assessment of HAHA were not collected in studies of adjuvant breast cancer.
The clinical relevance of the development of anti-trastuzumab antibodies after treatment with trastuzumab is not known.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Trastuzumab products have not been tested for carcinogenic potential.
No evidence of mutagenic activity was observed when trastuzumab was tested in the standard Ames bacterial and human peripheral blood lymphocyte mutagenicity assays, at concentrations of up to 5000 mcg/mL. In an in vivo micronucleus assay, no evidence of chromosomal damage to mouse bone marrow cells was observed following bolus intravenous doses of up to 118 mg/kg of trastuzumab.
A fertility study was conducted in female cynomolgus monkeys at doses up to 25 times the weekly recommended human dose of 2 mg/kg of trastuzumab and has revealed no evidence of impaired fertility, as measured by menstrual cycle duration and female sex hormone levels.
-
14 CLINICAL STUDIES
14.1 Adjuvant Breast Cancer
The safety and efficacy of trastuzumab in women receiving adjuvant chemotherapy for HER2 overexpressing breast cancer were evaluated in an integrated analysis of two randomized, open-label, clinical trials (Studies NSABP B31 and NCCTG N9831) with a total of 4063 women at the protocol-specified final overall survival analysis, a third randomized, open-label, clinical trial (HERA) with a total of 3386 women at definitive Disease-Free Survival analysis for one-year trastuzumab treatment versus observation, and a fourth randomized, open-label clinical trial with a total of 3222 patients (BCIRG006).
NSABP B31 and NCCTG N9831
In NSABP B31 and NCCTG N9831, breast tumor specimens were required to show HER2 overexpression (3+ by IHC) or gene amplification (by FISH). HER2 testing was verified by a central laboratory prior to randomization (NCCTG N9831) or was required to be performed at a reference laboratory (NSABP B31). Patients with a history of active cardiac disease based on symptoms, abnormal electrocardiographic, radiologic, or left ventricular ejection fraction findings or uncontrolled hypertension (diastolic > 100 mm Hg or systolic > 200 mm Hg) were not eligible.
Patients were randomized (1:1) to receive doxorubicin and cyclophosphamide followed by paclitaxel (AC → paclitaxel) alone or paclitaxel plus trastuzumab (AC → paclitaxel + trastuzumab). In both trials, patients received four 21-day cycles of doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2. Paclitaxel was administered either weekly (80 mg/m2) or every 3 eeks (175 mg/m2) for a total of 12 weeks in NSABP B31; paclitaxel was administered only by the weekly schedule in NCCTG N9831. Trastuzumab was administered at 4 mg/kg on the day of initiation of paclitaxel and then at a dose of 2 mg/kg weekly for a total of 52 weeks. Trastuzumab treatment was permanently discontinued in patients who developed congestive heart failure, or persistent/recurrent LVEF decline [see Dosage and Administration (2.5)]. Radiation therapy, if administered, was initiated after the completion of chemotherapy. Patients with ER+ and/or PR+ tumors received hormonal therapy. The major efficacy outcome measure of the combined efficacy analysis was Disease-Free Survival (DFS), defined as the time from randomization to recurrence, occurrence of contralateral breast cancer, other second primary cancer, or death. An additional efficacy outcome measure was was overall survival (OS).
A total of 3752 patients were included in the joint efficacy analysis of the primary endpoint of DFS following a median follow-up of 2.0 years in the AC → paclitaxel + trastuzumab arm. The pre-planned final OS analysis from the joint analysis included 4063 patients and was performed when 707 deaths had occurred after a median follow-up of 8.3 years in the AC → paclitaxel + trastuzumab arm. The data from both arms in NSABP B31 and two of the three study arms in NCCTG N9831 were pooled for efficacy analyses.
The patients included in the primary DFS analysis had a median age of 49 years (range, 22 to 80 years; 6% > 65 years), 84% were white, 7% black, 4% Hispanic, and 4% Asian/Pacific Islander. Disease characteristics included 90% infiltrating ductal histology, 38% T1, 91% nodal involvement, 27% intermediate and 66% high grade pathology, and 53% ER+ and/or PR+ tumors.
HERA
In HERA, breast tumor specimens were required to show HER2 overexpression (3+ by IHC) or gene amplification (by FISH) as determined at a central laboratory. Patients with node-negative disease were required to have ≥ T1c primary tumor. Patients with a history of congestive heart failure or LVEF < 55%, uncontrolled arrhythmias, angina requiring medication, clinically significant valvular heart disease, evidence of transmural infarction on ECG, poorly controlled hypertension (systolic > 180 mm Hg or diastolic > 100 mm Hg) were not eligible.
Patients were randomized (1:1:1) upon completion of definitive surgery, and at least four cycles of chemotherapy to receive no additional treatment, or one year of trastuzumab treatment or two years of trastuzumab treatment. Patients undergoing a lumpectomy had also completed standard radiotherapy. Patients with ER+ and/or PgR+ disease received systemic adjuvant hormonal therapy at investigator discretion. Trastuzumab was administered with an initial dose of 8 mg/kg followed by subsequent doses of 6 mg/kg once every three weeks. The major efficacy outcome measure was Disease-Free Survival (DFS), defined as in NSABP B31 and NCCTG N9831.
HERA was designed to compare one and two years of three-week trastuzumab treatment versus observation in patients with HER2 positive EBC following surgery, established chemotherapy and radiotherapy (if applicable). A protocol specified interim efficacy analysis comparing one-year trastuzumab treatment to observation was performed at a median follow-up duration of 12.6 months in the trastuzumab arm.
Among the 3386 patients randomized to the observation (n = 1693) and trastuzumab one-year (n = 1693) treatment arms, the median age was 49 years (range 21 to 80), 83% were White, and 13% were Asian. Disease characteristics: 94% infiltrating ductal carcinoma, 50% ER+ and/or PgR+, 57% node positive, 32% node negative, and in 11% of patients, nodal status was not assessable due to prior neo-adjuvant chemotherapy. Ninety-six percent (1055/1098) of patients with node-negative disease had high-risk features: among the 1098 patients with node-negative disease, 49% (543) were ER− and PgR−, and 47% (512) were ER and/or PgR + and had at least one of the following high-risk features: pathological tumor size greater than 2 cm, Grade 2 to 3, or age <35 years. Prior to randomization, 94% of patients had received anthracycline-based chemotherapy regimens.
After the DFS results comparing observation to one-year trastuzumab treatment were disclosed, a prospectively planned analysis that included comparison of one year versus two years of trastuzumab treatment at a median follow-up duration of 8 years was performed. Based on this analysis, extending trastuzumab treatment for a duration of two years did not show additional benefit over treatment for one year [Hazard Ratios of two-years trastuzumab versus one-year trastuzumab treatment in the intent to treat (ITT) population for Disease-Free Survival (DFS) = 0.99 (95% CI: 0.87, 1.13), p-value = 0.90 and Overall Survival (OS) = 0.98 (0.83, 1.15); p-value = 0.78].
BCIRG006
In BCIRG006, breast tumor specimens were required to show HER2 gene amplification (FISH+ only) as determined at a central laboratory. Patients were required to have either node-positive disease, or node-negative disease with at least one of the following high-risk features: ER/PR-negative, tumor size > 2 cm, age < 35 years, or histologic and/or nuclear Grade 2 or 3. Patients with a history of CHF, myocardial infarction, Grade 3 or 4 cardiac arrhythmia, angina requiring medication, clinically significant valvular heart disease, poorly controlled hypertension (diastolic > 100 mm Hg), any T4 or N2 or known N3 or M1 breast cancer were not eligible.
Patients were randomized (1:1:1) to receive doxorubicin and cyclophosphamide followed by docetaxel (AC-T), doxorubicin and cyclophosphamide followed by docetaxel plus trastuzumab (AC-TH), or docetaxel and carboplatin plus trastuzumab (TCH). In both the AC-T and AC-TH arms, doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 were administered every 3 weeks for four cycles; docetaxel 100 mg/m2 was administered every 3 weeks for four cycles. In the TCH arm, docetaxel 75 mg/m2 and carboplatin (at a target AUC of 6 mg/mL/min as a 30-to 60-minute infusion) were administered every 3 weeks for six cycles. Trastuzumab was administered weekly (initial dose of 4 mg/kg followed by weekly dose of 2 mg/kg) concurrently with either T or TC, and then every 3 weeks (6 mg/kg) as monotherapy for a total of 52 weeks. Radiation therapy, if administered, was initiated after completion of chemotherapy. Patients with ER+ and/or PR+ tumors received hormonal therapy. Disease-Free Survival (DFS) was the main outcome measure.
Among the 3222 patients randomized, the median age was 49 (range 22 to 74 years; 6% ≥ 65 years). Disease characteristics included 54% ER+ and/or PR+ and 71% node positive. Prior to randomization, all patients underwent primary surgery for breast cancer.
The results for DFS for the integrated analysis of NSABP B31 and NCCTG N9831, HERA, and BCIRG006 and OS results for the integrated analysis of NSABP B31 and NCCTG N9831, and HERA are presented in Table 9. For NSABP B31 and NCCTG N9831, the duration of DFS following a median follow-up of 2.0 years in the AC → TH arm is presented in Figure 4, and the duration of OS after a median follow-up of 8.3 years in the AC → TH arm is presented in Figure 5. The duration of DFS for BCIRG006is presented in Figure 6. For NSABP B31 and NCCTG N9831, the OS hazard ratio was 0.64 (95% CI: 0.55, 0.74). At 8.3 years of median follow-up [AC → TH], the survival rate was estimated to be 86.9% in the AC → TH arm and 79.4% in the AC → T arm. The final OS analysis results from NSABP B31 and NCCTG N9831 indicate that OS benefit by age, hormone receptor status, number of positive lymph nodes, tumor size and grade, and surgery/radiation therapy was consistent with the treatment effect in the overall population. In patients ≤ 50 years of age (n = 2197), the OS hazard ratio was 0.65 (95% CI: 0.52, 0.81) and in patients > 50 years of age (n = 1866), the OS hazard ratio was 0.63 (95% CI: 0.51, 0.78). In the subgroup of patients with hormone receptor-positive disease (ER-positive and/or PR-positive) (n = 2223), the hazard ratio for OS was 0.63 (95% CI: 0.51, 0.78). In the subgroup of patients with hormone receptor-negative disease (ER-negative and PR-negative) (n = 1830), the hazard ratio for OS was 0.64 (95% CI: 0.52, 0.80). In the subgroup of patients with tumor size ≤ 2 cm (n = 1604), the hazard ratio for OS was 0.52 (95% CI: 0.39, 0.71). In the subgroup of patients with tumor size > 2 cm (n = 2448), the hazard ratio for OS was 0.67 (95% CI: 0.56, 0.80).
Table 9
Efficacy Results from Adjuvant Treatment of Breast Cancer (NSABP B31 and NCCTG N9831, HERA, and BCIRG006)
DFS
events
DFS Hazard
ratio
(95% CI)
p-value
Deaths
(OS events)
OS Hazard
ratio
p-value
NSABP B31 and NCCTG N9831a AC → TH
(n = 1872)b
(n = 2031)c
133b 0.48b,d
(0.39, 0.59)
p < 0.0001e
289c 0.64c,d
(0.55, 0.74)
p < 0.0001e
AC → T
(n = 1880)b
(n = 2032)c
261b 418c HERAf Chemo →
Trastuzumab
(n = 1693)
127 0.54
(0.44, 0.67)
p < 0.0001g
31 0.75
p = NSh
Chemo →
Observation
(n = 1693)
219 40 BCIRG006i TCH
(n = 1075)
134 0.67
(0.54 to 0.84)
p = 0.0006e,j
56 AC → TH
(n = 1074)
121 0.60
(0.48 to 0.76)
p < 0.0001e,i
49 AC → T
(n = 1073)
180 80 CI = confidence interval.
a NSABP B31 and NCCTG N9831 regimens: doxorubicin and cyclophosphamide followed by paclitaxel (AC → T) or paclitaxel plus trastuzumab (AC → TH).
b Efficacy evaluable population, for the primary DFS analysis, following a median follow-up of 2.0 years in the AC → TH arm.
c Efficacy evaluable population, for the final OS analysis, following 707 deaths (8.3 years of median follow-up in the AC → TH arm).
d Hazard ratio estimated by Cox regression stratified by clinical trial, intended paclitaxel schedule, number of positive nodes, and hormone receptor status.
e stratified log-rank test.
f At definitive DFS analysis with median duration of follow-up of 12.6 months in the one-year trastuzumab treatment arm.
g log-rank test.
h NS = non-significant.
i BCIRG006 regimens: doxorubicin and cyclophosphamide followed by docetaxel (AC → T) or docetaxel plus trastuzumab (AC → TH); docetaxel and carboplatin plus trastuzumab (TCH).
j two-sided alpha level of 0.025 for each comparison.
Figure 4
Duration of Disease-Free Survival in Patients with Adjuvant Treatment of Breast Cancer (NSABP B31 and NCCTG N9831)
Figure 5
Duration of Overall Survival in Patients with Adjuvant Treatment of Breast Cancer (NSABP B31 and NCCTG N9831)
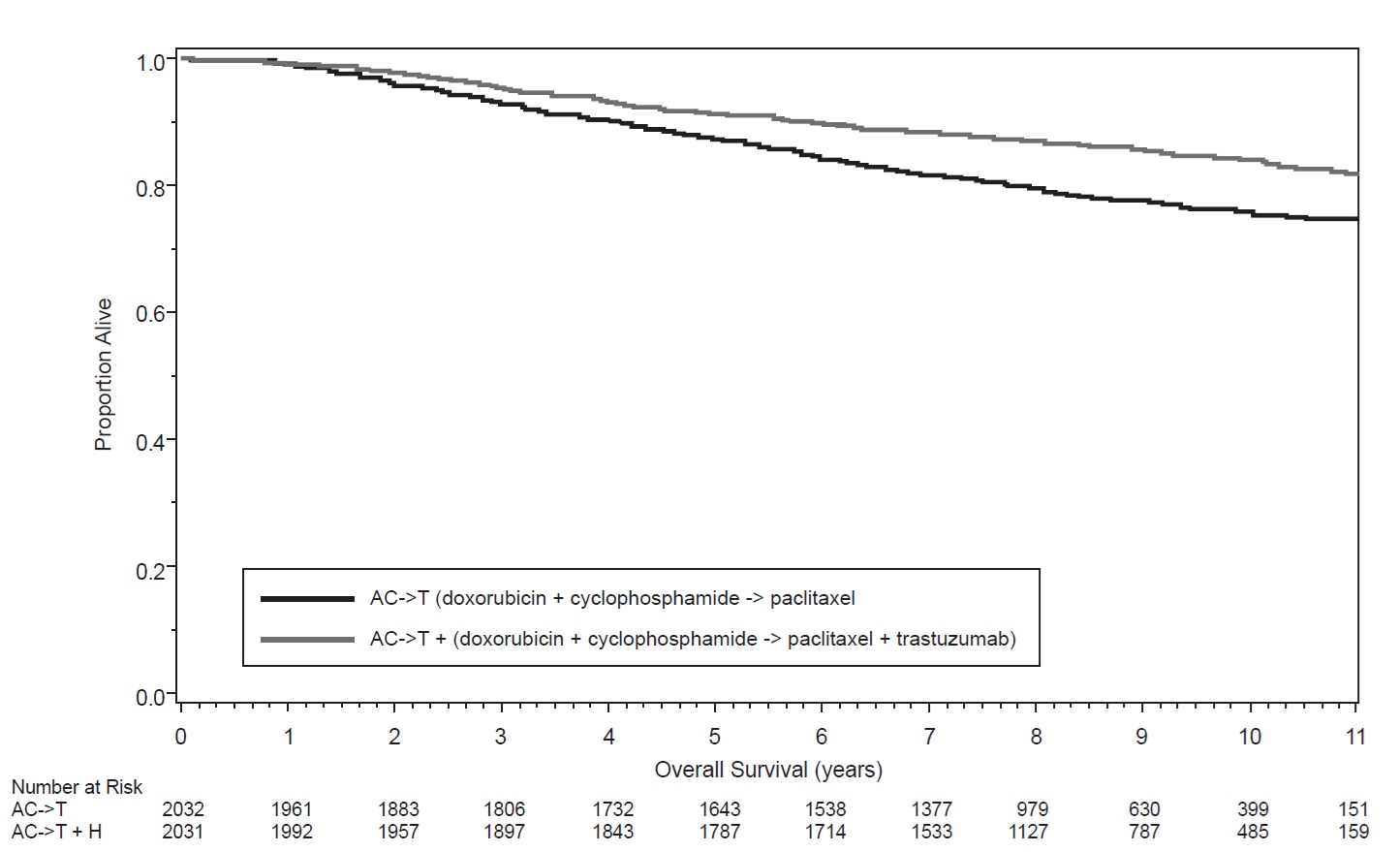
Figure 6
Duration of Disease-Free Survival in Patients with Adjuvant Treatment of Breast Cancer (BCIRG006)
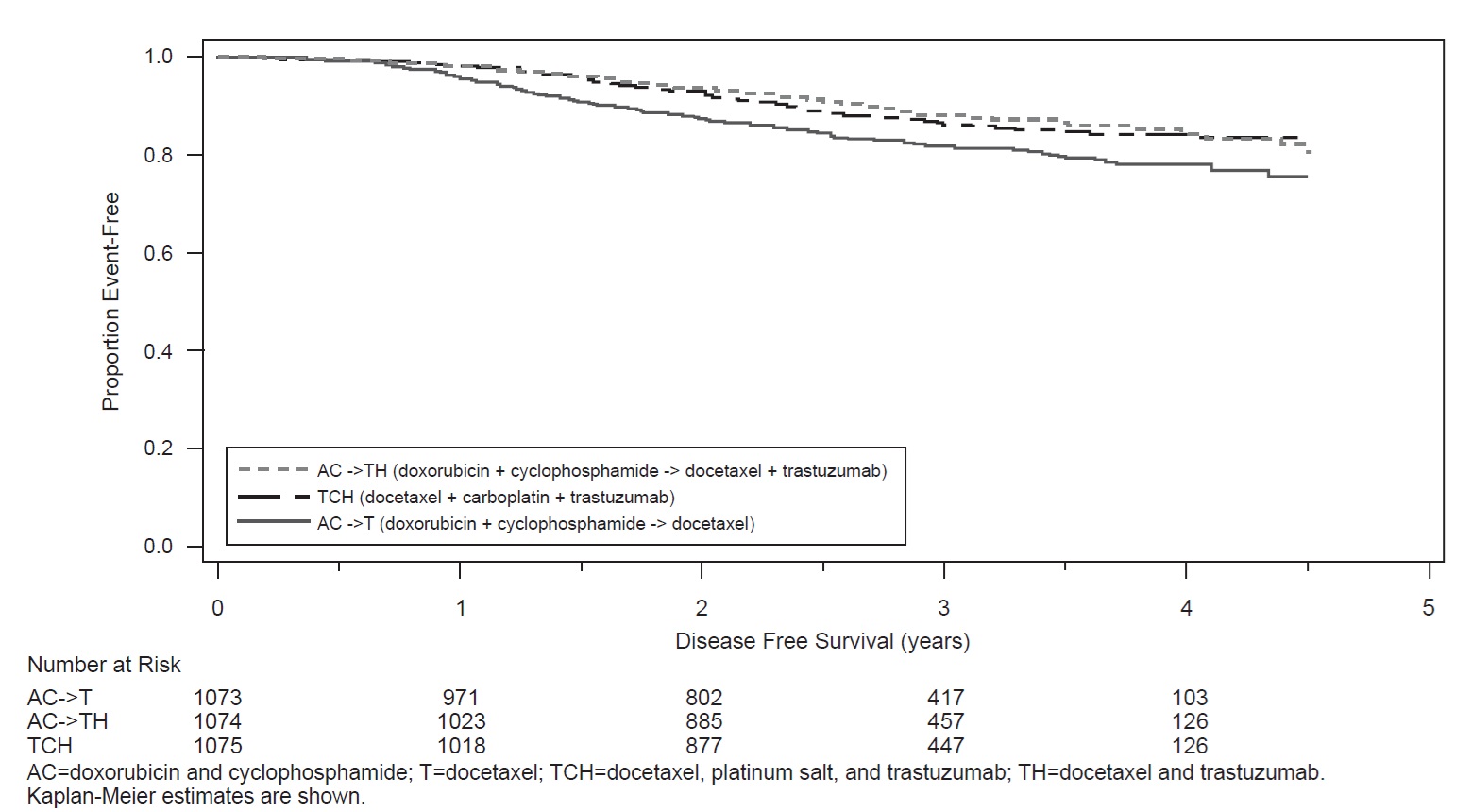
Exploratory analyses of DFS as a function of HER2 overexpression or gene amplification were conducted for patients in Studies 2 and 3, where central laboratory testing data were available. The results are shown in Table 10. The number of events in NCCTG N9831 was small with the exception of the IHC 3+/FISH+ subgroup, which constituted 81% of those with data. Definitive conclusions cannot be drawn regarding efficacy within other subgroups due to the small number of events. The number of events in HERA was adequate to demonstrate significant effects on DFS in the IHC 3+/FISH unknown and the FISH +/IHC unknown subgroups.
Table 10:DFS in NCCTG N9831 and HERA for Patients with HER2 Overexpression or Amplification
NCCTG N9831 HERAc HER2 Assay
Resulta
Number of
Patients
Hazard Ratio DFS
(95% CI)
Number of
Patients
Hazard Ratio DFS
(95% CI)
IHC 3+ FISH (+)
1170
0.42
(0.27, 0.64)
91 0.56
(0.13, 2.50)
FISH (–)
51 0.71
(0.04, 11.79)
8 — FISH Unknown 51 0.69
(0.09, 5.14)
2258 0.53
(0.41, 0.69)
IHC < 3+ /
FISH (+)
174 1.01
(0.18, 5.65)
299b 0.53
(0.20, 1.42)
IHC unknown /
FISH (+)
— — 724 0.59
(0.38, 0.93)
a IHC by HercepTest, FISH by PathVysion (HER2/CEP17 ratio ≥ 2.0) as performed at a central laboratory.
b All cases in this category in HERA were IHC 2+.
c Median follow-up duration of 12.6 months in the one-year trastuzumab treatment arm.
14.2 Metastatic Breast Cancer
The safety and efficacy of trastuzumab in treatment of women with metastatic breast cancer were studied in a randomized, controlled clinical trial in combination with chemotherapy (H0648g, n = 469 patients) and an open-label single agent clinical trial (H0649g, n = 222 patients). Both trials studied patients with metastatic breast cancer whose tumors overexpress the HER2 protein. Patients were eligible if they had 2 or 3 levels of overexpression (based on a 0 to 3 scale) by immunohistochemical assessment of tumor tissue performed by a central testing lab.
Previously Untreated Metastatic Breast Cancer (H0648g)
H0648g was a multicenter, randomized, open-label clinical trial conducted in 469 women with metastatic breast cancer who had not been previously treated with chemotherapy for metastatic disease. Tumor specimens were tested by IHC (Clinical Trial Assay, CTA) and scored as 0, 1+, 2+, or 3+, with 3+ indicating the strongest positivity. Only patients with 2+ or 3+ positive tumors were eligible (about 33% of those screened). Patients were randomized to receive chemotherapy alone or in combination with trastuzumab given intravenously as a 4 mg/kg loading dose followed by weekly doses of trastuzumab at 2 mg/kg. For those who had received prior anthracycline therapy in the adjuvant setting, chemotherapy consisted of paclitaxel (175 mg/m2 over 3 hours every 21 days for at least six cycles); for all other patients, chemotherapy consisted of anthracycline plus cyclophosphamide (AC: doxorubicin 60 mg/m2 or epirubicin 75 mg/m2 plus 600 mg/m2 cyclophosphamide every 21 days for six cycles). Sixty-five percent of patients randomized to receive chemotherapy alone in this study received trastuzumab at the time of disease progression as part of a separate extension study.
Based upon the determination by an independent response evaluation committee the patients randomized to trastuzumab and chemotherapy experienced a significantly longer median time to disease progression, a higher overall response rate (ORR), and a longer median duration of response, as compared with patients randomized to chemotherapy alone. Patients randomized to trastuzumab and chemotherapy also had a longer median survival (see Table 11).
These treatment effects were observed both in patients who received trastuzumab plus paclitaxel and in those who received trastuzumab plus AC; however the magnitude of the effects was greater in the paclitaxel subgroup.
Table 11
H0648g: Efficacy Results inFirst-Line Treatment for Metastatic Breast Cancer
Combined Results Paclitaxel Subgroup AC Subgroup trastuzumab +
All Chemo-
therapy
(n = 235)
All Chemo-
therapy
(n = 234)
trastuzumab +
Paclitaxel
(n = 92)
Paclitaxel
(n = 96)
trastuzumab +
ACa
(n = 143)
AC
(n = 138)
Time to Disease Progression (TTP) Median
TTP(mos)b,c
7.2 4.5 6.7 2.5 7.6 5.7 95% CI 7, 8 4, 5 5, 10 2, 4 7, 9 5, 7 p-valued < 0.0001 < 0.0001 0.002 Overall Response Rate (ORR)b Events (n) 45 29 38 15 50 38 95% CI 39, 51 23, 35 28, 48 8, 22 42, 58 30, 46 p-valuee < 0.001 < 0.001 0.10 Duration of Response (DoR) Median (months)b,c 8.3 5.8 8.3 4.3 8.4 6.4 25%, 75%
Quartile
6, 15 4, 8 5, 11 4, 7 6, 15 4, 8 Overall Survival (OS) Median (months)c 25.1 20.3 22.1 18.4 26.8 21.4 95% CI 22, 30 17, 24 17, 29 13, 24 23, 33 18, 27 p-valued 0.05 0.17 0.16 a AC = Anthracycline (doxorubicin or epirubicin) and cyclophosphamide.
b Assessed by an independent Response Evaluation Committee.
c Kaplan-Meier Estimate.
d log-rank test.
e χ2-test.
Data from H0648g suggest that the beneficial treatment effects were largely limited to patients with the highest level of HER2 protein overexpression (3+) (see Table 12).
Table 12
Treatment Effects in H0648g as aFunction of HER2 Overexpression or Amplification
HER2 Assay
Result
Number of
Patients
(N)
Relative Riskb for Time to
Disease Progression
(95% CI)
Relative Riskb for
Mortality
(95% CI)
CTA 2+ or 3+ 469 0.49 (0.40, 0.61) 0.80 (0.64, 1.00) FISH (+)a 325 0.44 (0.34, 0.57) 0.70 (0.53, 0.91) FISH (−)a 126 0.62 (0.42, 0.94) 1.06 (0.70, 1.63) CTA 2+ 120 0.76 (0.50, 1.15) 1.26 (0.82, 1.94) FISH (+) 32 0.54 (0.21, 1.35) 1.31 (0.53, 3.27) FISH (−) 83 0.77 (0.48, 1.25) 1.11 (0.68, 1.82) CTA 3+ 349 0.42 (0.33, 0.54) 0.70 (0.51, 0.90) FISH (+) 293 0.42 (0.32, 0.55) 0.67 (0.51, 0.89) FISH (−) 43 0.43 (0.20, 0.94) 0.88 (0.39, 1.98) a FISH testing results were available for 451 of the 469 patients enrolled on study.
b The relative risk represents the risk of progression or death in the trastuzumab plus chemotherapy arm versus the chemotherapy arm.
Previously Treated Metastatic Breast Cancer (H0649g)
Trastuzumab was studied as a single agent in a multicenter, open-label, single-arm clinical trial (H0649g) in patients with HER2 overexpressing metastatic breast cancer who had relapsed following one or two prior chemotherapy regimens for metastatic disease. Of 222 patients enrolled, 66% had received prior adjuvant chemotherapy, 68% had received two prior chemotherapy regimens for metastatic disease, and 25% had received prior myeloablative treatment with hematopoietic rescue. Patients were treated with a loading dose of 4 mg/kg IV followed by weekly doses of trastuzumab at 2 mg/kg IV.
The ORR (complete response + partial response), as determined by an independent Response Evaluation Committee, was 14%, with a 2% complete response rate and a 12% partial response rate. Complete responses were observed only in patients with disease limited to skin and lymph nodes.
The overall response rate in patients whose tumors tested as CTA 3+ was 18% while in those that tested as CTA 2+, it was 6%.
14.3 Metastatic Gastric Cancer
The safety and efficacy of trastuzumab in combination with cisplatin and a fluoropyrimidine (capecitabine or 5-fluorouracil) were studied in patients previously untreated for metastatic gastric or gastroesophageal junction adenocarcinoma (ToGA). In this open-label, multi-center trial, 594 patients were randomized 1:1 to trastuzumab in combination with cisplatin and a fluoropyrimidine (FC+H) or chemotherapy alone (FC). Randomization was stratified by extent of disease (metastatic vs. locally advanced), primary site (gastric vs. gastroesophageal junction), tumor measurability (yes vs. no), ECOG performance status (0,1 vs. 2), and fluoropyrimidine (capecitabine vs. 5-fluorouracil). All patients were either HER2 gene amplified (FISH+) or HER2 overexpressing (IHC 3+). Patients were also required to have adequate cardiac function (e.g., LVEF > 50%).
On the trastuzumab-containing arm, trastuzumab was administered as an IV infusion at an initial dose of 8 mg/kg followed by 6 mg/kg every 3 weeks until disease progression. On both study arms cisplatin was administered at a dose of 80 mg/m2 Day 1 every 3 weeks for 6 cycles as a 2 hour IV infusion. On both study arms capecitabine was administered at 1000 mg/m2 dose orally twice daily (total daily dose 2000 mg/m2) for 14 days of each 21 day cycle for 6 cycles. Alternatively, continuous intravenous infusion (CIV) 5-fluorouracil was administered at a dose of 800 mg/m2/day from Day 1 through Day 5 every three weeks for 6 cycles.
The median age of the study population was 60 years (range: 21 to 83); 76% were male; 53% were Asian, 38% Caucasian, 5% Hispanic, 5% other racial/ethnic groups; 91% had ECOG PS of 0 or 1; 82% had primary gastric cancer and 18% had primary gastroesophageal adenocarcinoma. Of these patients, 23% had undergone prior gastrectomy, 7% had received prior neoadjuvant and/or adjuvant therapy, and 2% had received prior radiotherapy.
The main outcome measure of ToGA was overall survival (OS), analyzed by the unstratified logrank test. The final OS analysis based on 351 deaths was statistically significant (nominal significance level of 0.0193). An updated OS analysis was conducted at one year after the final analysis. The efficacy results of both the final and the updated analyses are summarized in Table 13 and Figure 7.
Table 13: Overall Survival in ToGA (ITT Population) FCa + Trastuzumab Arm
N = 298
FCa Arm
N = 296
Overall Survival (interim analysis) N (%)
Median (months)
95% CI
167 (56.0%)
13.5
(11.7, 15.7)
184 (62.2%)
11.0
(9.4, 12.5)
Hazard Ratio
95% CI
p-valueb
0.73
(0.60, 0.91)
0.0038
Overall Survival (updated) N (%)
Median (months)
95% CI
221 (74.2%)
13.1
(11.9, 15.1)
227 (76.7%)
11.7
(10.3, 13.0)
Hazard Ratio
95% CI
0.80
(0.67, 0.97)
a FC = capecitabine vs. 5-fluorouracil
b Two sided p-value comparing with the nominal significance level of 0.0193.
Figure 7
Updated Overall Survival in Patients with Metastatic Gastric Cancer (ToGA)
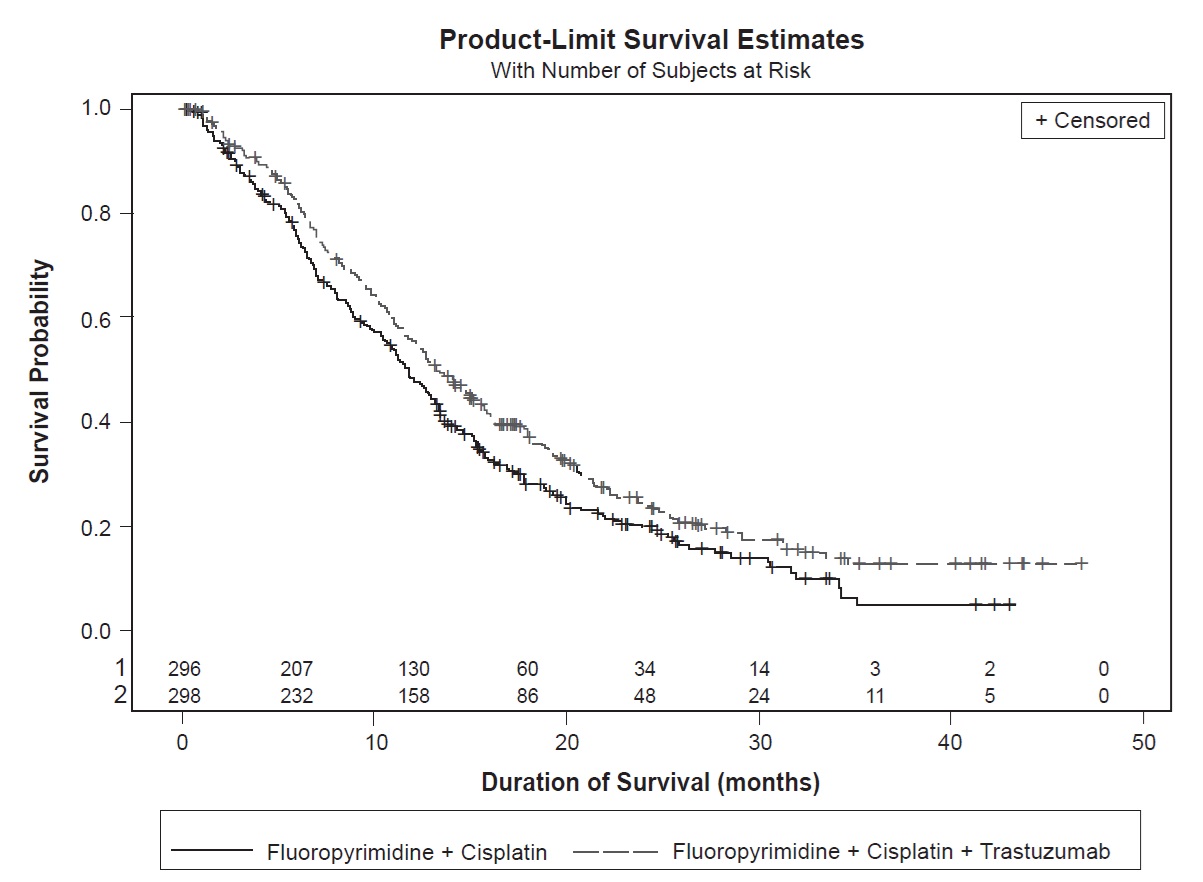
An exploratory analysis of OS in patients based on HER2 gene amplification (FISH) and protein overexpression (IHC) testing is summarized in Table 14.
Table 14
Exploratory analyses by HER2 Status Using Updated Overall survival Results
FC
(N = 296)a
FC + H
(N = 298)b
FISH+ / IHC 0, 1+ subgroup (N = 133) No. Deaths (%) / n (%) 57/71 (80%) 56/62 (90%) Median OS Duration (mos.) 8.8 8.3 95% CI (mos.) (6.4, 11.7) (6.2, 10.7) Hazard Ratio (95% CI) 1.33 (0.92, 1.92) FISH+ / IHC2+ subgroup (N = 160) No. Deaths (%) / n (%) 65/80 (81%) 64/80 (80%) Median OS Duration (mos.) 10.8 12.3 95% CI (mos.) (6.8, 12.8) (9.5, 15.7) Hazard Ratio (95% CI) 0.78 (0.55, 1.10) FISH+ or FISH-/ IHC3+c subgroup (N = 294) No. Deaths (%) / n (%) 104/143 (73%) 96/151 (64%) Median OS Duration (mos.) 13.2 18.0 95% CI (mos.) (11.5, 15.2) (15.5, 21.2) Hazard Ratio (95% CI) 0.66 (0.50, 0.87) a Two patients on the FC arm who were FISH+ but IHC status unknown were excluded from the exploratory subgroup analyses.
b Five patients on the trastuzumab-containing arm who were FISH+, but IHC status unknown were excluded from the exploratory subgroup analyses.
c Includes 6 patients on chemotherapy arm, 10 patients on trastuzumab arm with FISH–, IHC3+ and 8 patients on chemotherapy arm, 8 patients on trastuzumab arm with FISH status unknown, IHC 3+.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Ogivri (trastuzumab-dkst) for injection 420 mg/vial is supplied in a multiple-dose vial as an off-white to pale yellow lyophilized sterile powder, under vacuum. Each carton contains one multiple-dose vial of Ogivri and one vial (20 mL) of Bacteriostatic Water for Injection (BWFI), USP, containing 1.1% benzyl alcohol as a preservative.
NDC 83257-004-12
Ogivri (trastuzumab-dkst) for injection 420 mg/vial is supplied in a multiple-dose vial as an off-white to pale yellow lyophilized sterile powder, under vacuum. Each carton contains one multiple-dose vial of Ogivri. No diluent is provided.
NDC 83257-003-01
Ogivri (trastuzumab-dkst) for injection 150 mg/vial is supplied in a single-dose vial as an off-white to pale yellow lyophilized sterile powder, under vacuum. Each carton contains one single-dose vial of Ogivri.
NDC 83257-001-11
Store Ogivri vials in the refrigerator at 2° to 8°C (36° to 46°F) until time of reconstitution.
-
17 PATIENT COUNSELING INFORMATION
Cardiomyopathy
-
Advise patients to contact a health care professional immediately for any of the following: new onset or worsening shortness of breath, cough, swelling of the ankles/legs, swelling of the face, palpitations, weight gain of more than 5 pounds in 24 hours, dizziness or loss of consciousness [see Boxed Warning: Cardiomyopathy].
Embryo-Fetal Toxicity
-
Advise pregnant women and females of reproductive potential that Ogivri exposure during pregnancy or within 7 months prior to conception can result in fetal harm. Advise female patients to contact their healthcare provider with a known or suspected pregnancy [see Use in Specific Populations (8.1)].
-
Advise females of reproductive potential to use effective contraception during treatment and for 7 months following the last dose of Ogivri [see Use in Specific Populations (8.3)].
Ogivri® is a registered trademark of Biosimilars New Co. Ltd.; a Biocon Biologics Company.
Copyright © 2023 Biocon Biologics Inc. All rights reserved.
Manufactured by and for:
Biocon Biologics Inc.
245 Main st, 2nd floor
Cambridge, MA 02142, U.S.A.
U.S Licence No. 2324
KR/DRUGS/KTK/28D/07/2006
-
-
PRINCIPAL DISPLAY PANEL - 420 mg/vial with Diluent
NDC 83257-004-12
Rx only
Ogivri®
(trastuzumab-dkst)
For Injection
420 mg/vialFor intravenous infusion after reconstitution
No preservative
KEEP REFRIGERATED
Multiple-Dose
VialContents: Each carton contains one 420 mg vial of Ogivri and one vial containing 20 mL of Bacteriostatic Water for Injection, USP (1.1% benzyl alcohol).
The content of each Ogivri® vial is 420 mg trastuzumab-dkst, D-sorbitol (322.6 mg), L-Histidine (6.0 mg), L-Histidine hydrochloride monohydrate (9.4 mg) and Polyethylene glycol 3350/Macrogol 3350 (94.1 mg). Hydrochloric acid or sodium hydroxide may be used to adjust the pH of the formulation buffer.
No U.S. standard of potency.
Bacteriostatic Water for Injection, USP is a sterile water containing 1.1% benzyl alcohol as an antimicrobial preservative packaged in a multi-use vial. The diluent is pH 4.5 to 7.
Store in refrigerator at 2° to 8°C (36° to 46°F) until time of reconstitution.
Reconstitution, Dosage, and Administration: Do not use if vacuum is not present. See prescribing information for dosage, preparation, and administration. Reconstitute with 20 mL Bacteriostatic Water for Injection (BWFI), USP (0.9% to 1.1% benzyl alcohol) to yield a multiple-dose solution containing 21 mg/mL trastuzumab-dkst that delivers 20 mL (420 mg trastuzumab-dkst). Do Not Shake. Store reconstituted solution in refrigerator at 2° to 8°C (36° to 46°F). Do not Freeze. Discard unused reconstituted solution after 28 days.
Do Not Freeze.
Do Not Shake Reconstituted Solution.
KEEP REFRIGERATED
Ogivri® and the Ogivri Logo are registered trademarks of Biosimilars New Co. Ltd.; a Biocon Biologics Company.
Copyright © 2023 Biocon Biologics Inc. All rights reservedy.
Manufactured by:
Biocon Biologics Inc.
245 Main st, 2nd floor
Cambridge, MA 02142, U.S.A.
U.S Licence No. 2324
Product of India
KR/DRUGS/KTK/28D/07/2006
KR/DRUGS/KTK/28/0456/2019
-
PRINCIPAL DISPLAY PANEL - 420 mg/vial
NDC 83257-003-01 Rx only
Ogivri®
(trastuzumab-dkst)
For Injection
420 mg/vialFor intravenous infusion after reconstitution
No preservative
KEEP REFRIGERATED
Multiple-Dose
VialContents: Each carton contains one 420 mg vial of Ogivri.
The content of each Ogivri® vial is 420 mg trastuzumab-dkst, D-sorbitol (322.6 mg), L-Histidine (6.0 mg), L-Histidine hydrochloride monohydrate (9.4 mg) and Polyethylene glycol 3350/Macrogol 3350 (94.1 mg). Hydrochloric acid or sodium hydroxide may be used to adjust the pH of the formulation buffer.
No U.S. standard of potency.
Store in refrigerator at 2° to 8°C (36° to 46°F) until time of reconstitution.
Reconstitution, Dosage, and Administration: Do not use if vacuum is not present. See prescribing information for dosage, preparation, and administration. Reconstitute with 20 mL Bacteriostatic Water for Injection (BWFI), USP (0.9% to 1.1% benzyl alcohol) to yield a multiple-dose solution containing 21 mg/mL trastuzumab-dkst that delivers 20 mL (420 mg trastuzumab-dkst). Do Not Shake. Store reconstituted solution in refrigerator at 2° to 8°C (36° to 46°F). Do not Freeze. Discard unused reconstituted solution after 28 days.
Do Not Freeze. Do Not Shake Reconstituted
Solution. KEEP REFRIGERATED
Ogivri® & the Ogivri Logo are registered trademarks of Biosimilars New Co. Ltd.; a Biocon Biologics Company.
Copyright © 2023 Biocon Biologics Inc.
All rights reserved.
Manufactured by:
Biocon Biologics Inc.
245 Main st, 2nd floor
Cambridge, MA 02142, U.S.A.
U.S Licence No. 2324
Product of IndiaKR/DRUGS/KTK/28D/07/2006
-
PRINCIPAL DISPLAY PANEL -150 mg/vial
NDC 83257-001-11
Rx Only
Ogivri®
(trastuzumab-dkst)
For Injection
150 mg/vialFor intravenous infusion after reconstitution
No preservative
KEEP REFRIGERATED
Single-Dose Vial
Discard Unused
PortionThe content of each Ogivri® vial is 150 mg trastuzumab-dkst, D-sorbitol (115.2 mg), L-Histidine (2.16 mg), L-Histidine hydrochloride monohydrate (3.36 mg) and Polyethylene glycol 3350/Macrogol 3350 (33.6 mg). Hydrochloric acid or sodium hydroxide may be used to adjust the pH of the formulation buffer.
No U.S. standard of potency.
Usual dosage: See package insert for dosage, preparation, and administration information.
Storage: Refrigerate at 2° to 8°C (36° to 46°F) until time of reconstitution.
Reconstitute immediately before use.
Do Not Freeze. Do Not Shake After Reconstitution.
Ogivri® & the Ogivri Logo are registered trademarks of Biosimilars New Co. Ltd.; a Biocon Biologics Company.
Copyright © 2023 Biocon Biologics Inc.
All rights reserved.
Manufactured by:
Biocon Biologics Inc.
245 Main st, 2nd floor
Cambridge, MA 02142, U.S.A
U.S Licence No. 2324
Product of IndiaKR/DRUGS/KTK/28D/07/2006
-
INGREDIENTS AND APPEARANCE
OGIVRI
trastuzumab-dkst kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:83257-004 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83257-004-12 1 in 1 CARTON; Type 0: Not a Combination Product 10/01/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, MULTI-DOSE 20 mL Part 2 1 VIAL 20 mL Part 1 of 2 OGIVRI
trastuzumab-dkst injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:83257-003 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRASTUZUMAB (UNII: P188ANX8CK) (TRASTUZUMAB - UNII:P188ANX8CK) TRASTUZUMAB 420 mg in 20 mL Inactive Ingredients Ingredient Name Strength HISTIDINE MONOHYDROCHLORIDE (UNII: 1D5Q932XM6) 9.4 mg in 20 mL HISTIDINE (UNII: 4QD397987E) 6.0 mg in 20 mL POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) 94.1 mg in 20 mL SORBITOL (UNII: 506T60A25R) 322.6 mg in 20 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83257-003-11 20 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761074 10/01/2023 Part 2 of 2 BACTERIOSTATIC WATER
bacteriostatic water solutionProduct Information Item Code (Source) NDC:83257-002 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) 11 mg in 20 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83257-002-11 20 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761074 10/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761074 10/01/2023 OGIVRI
trastuzumab-dkst injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:83257-003 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRASTUZUMAB (UNII: P188ANX8CK) (TRASTUZUMAB - UNII:P188ANX8CK) TRASTUZUMAB 420 mg in 20 mL Inactive Ingredients Ingredient Name Strength HISTIDINE MONOHYDROCHLORIDE (UNII: 1D5Q932XM6) 9.4 mg in 20 mL HISTIDINE (UNII: 4QD397987E) 6.0 mg in 20 mL POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) 94.1 mg in 20 mL SORBITOL (UNII: 506T60A25R) 322.6 mg in 20 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83257-003-01 1 in 1 CARTON 10/01/2023 10/09/2024 1 20 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761074 10/01/2023 OGIVRI
trastuzumab-dkst injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:83257-001 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRASTUZUMAB (UNII: P188ANX8CK) (TRASTUZUMAB - UNII:P188ANX8CK) TRASTUZUMAB 150 mg in 7.4 mL Inactive Ingredients Ingredient Name Strength HISTIDINE MONOHYDROCHLORIDE (UNII: 1D5Q932XM6) HISTIDINE (UNII: 4QD397987E) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) SORBITOL (UNII: 506T60A25R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83257-001-11 1 in 1 CARTON 10/01/2023 1 15 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761074 10/01/2023 Labeler - Biocon Biologics Inc. (117609395) Establishment Name Address ID/FEI Business Operations Biocon Biologics Limited 650501997 analysis(83257-004, 83257-002, 83257-003, 83257-001) , manufacture(83257-004, 83257-002, 83257-003, 83257-001) , pack(83257-004, 83257-002, 83257-003, 83257-001)



