Label: STERILE SALINE injection, solution
- NDC Code(s): 50989-885-15, 50989-885-16, 50989-885-17
- Packager: Vedco
- Category: PRESCRIPTION ANIMAL DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 12, 2019
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- WARNING:
- STORAGE
- KEEP OUT OF REACH OF CHILDREN
- CAUTION:
- INDICATIONS:
- DOSAGE
- INFORMATION FOR OWNERS/CAREGIVERS
-
PRINCIPAL DISPLAY PANEL
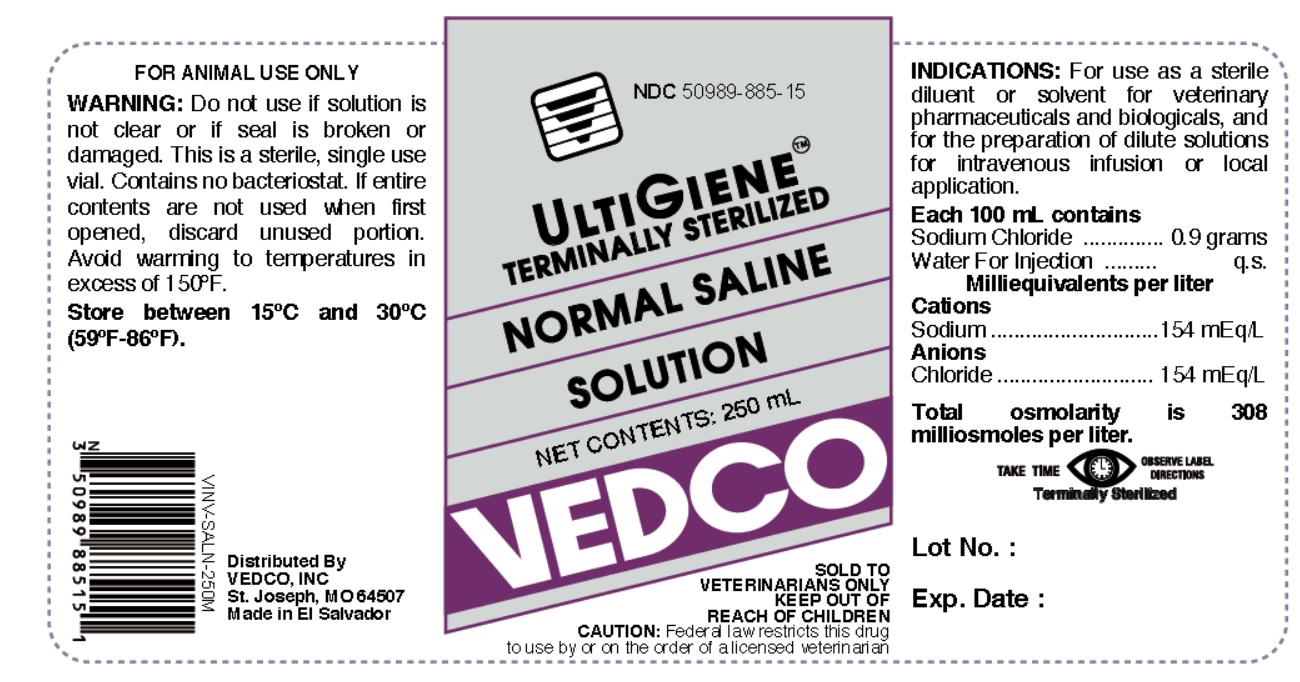
NDC 50989-885-15
ULTIGIENE TERMINALLY STERILIZED
NORMAL SALINE SOLUTION
NET CONTENTS: 250 mL
VEDCO
SOLD TO VETERINARIANS ONLY
KEEP OUT OF REACH OF CHILDREN
CAUTION: Federal Law (U.S.A) restricts this drug to use by or on the order of a licensed veterinarian
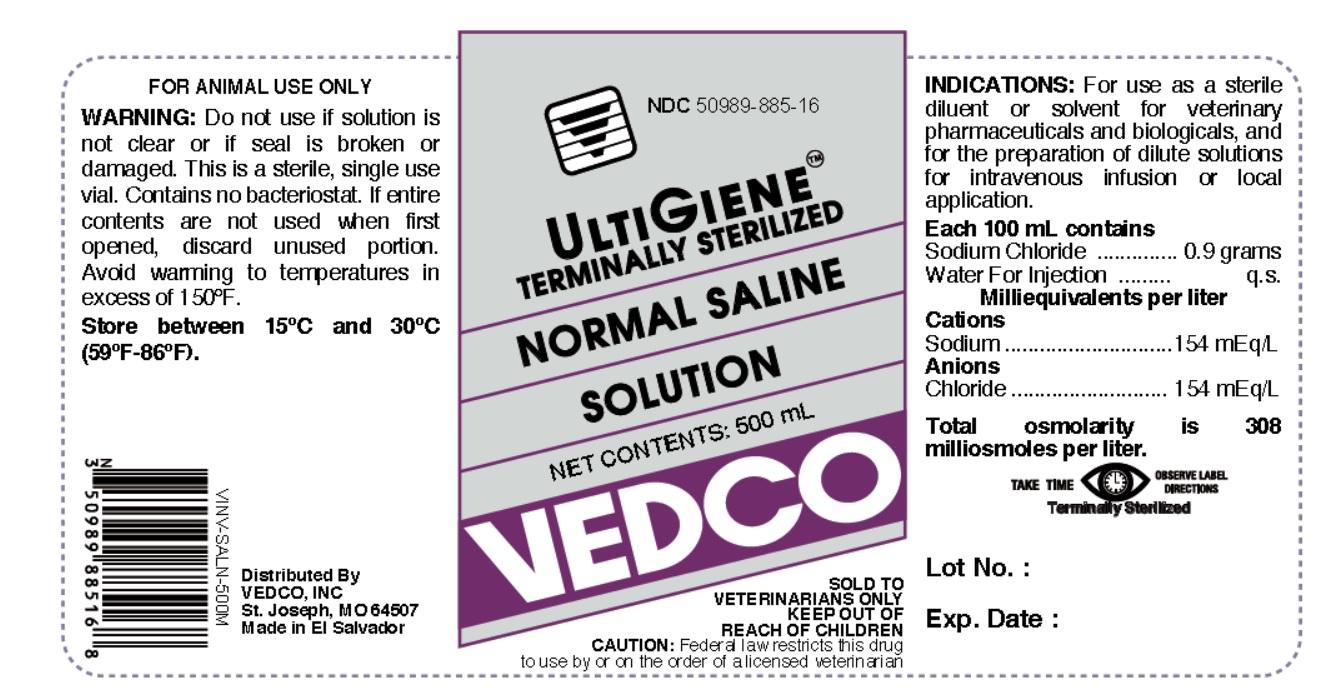
NDC 50989-885-16
ULTIGIENE TERMINALLY STERILIZED
NORMAL SALINE SOLUTION
NET CONTENTS: 500 mL
VEDCO
SOLD TO VETERINARIANS ONLY
KEEP OUT OF REACH OF CHILDREN
CAUTION: Federal Law (U.S.A) restricts this drug to use by or on the order of a licensed veterinarian
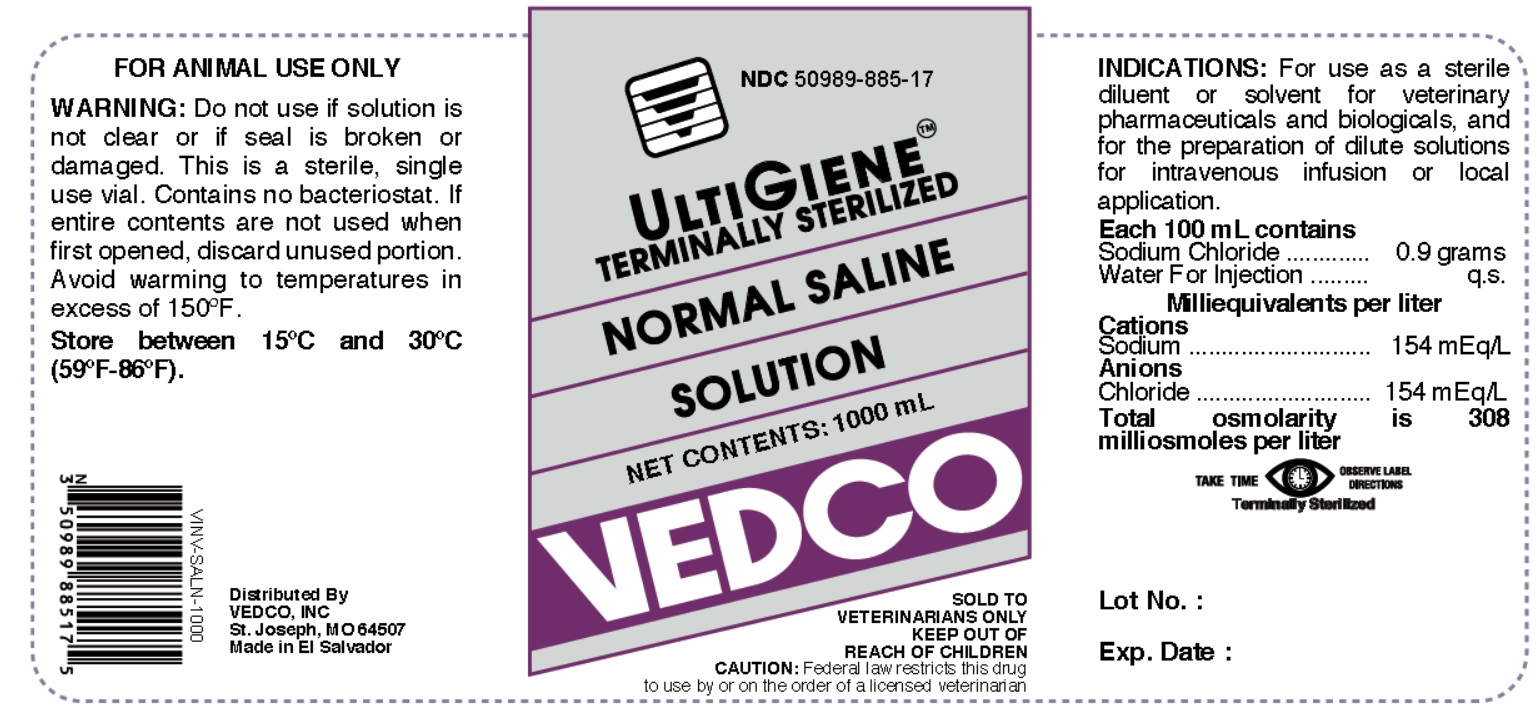
NDC 50989-885-17
ULTIGIENE TERMINALLY STERILIZED
NORMAL SALINE SOLUTION
NET CONTENTS: 1000 mL
VEDCO
SOLD TO VETERINARIANS ONLY
KEEP OUT OF REACH OF CHILDREN
CAUTION: Federal Law (U.S.A) restricts this drug to use by or on the order of a licensed veterinarian
-
INGREDIENTS AND APPEARANCE
STERILE SALINE
sterile saline injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:50989-885 Route of Administration INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 0.9 g in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50989-885-17 1000 mL in 1 BOTTLE, PLASTIC 2 NDC:50989-885-16 500 mL in 1 BOTTLE, PLASTIC 3 NDC:50989-885-15 250 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/09/2019 Labeler - Vedco (021634266) Registrant - Vedco (021634266)



