Label: DIPHTHERIA AND TETANUS TOXOIDS ADSORBED (corynebacterium diphtheriae toxoid antigen (formaldehyde inactivated) and clostridium tetani toxoid antigen- formaldehyde inactivated injection, suspension
- NDC Code(s): 49281-225-10, 49281-225-58
- Packager: Sanofi Pasteur Inc.
- Category: VACCINE LABEL
Drug Label Information
Updated April 19, 2019
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Diphtheria and Tetanus Toxoids Adsorbed safely and effectively. See full prescribing information for Diphtheria and Tetanus Toxoids Adsorbed.
Diphtheria and Tetanus Toxoids Adsorbed
Suspension for Intramuscular Injection
Initial U.S. Approval: 1997INDICATIONS AND USAGE
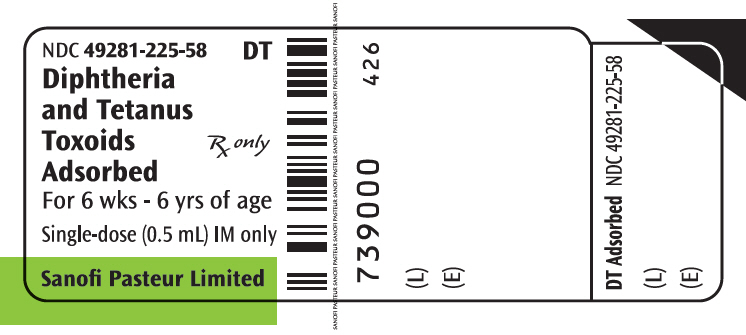
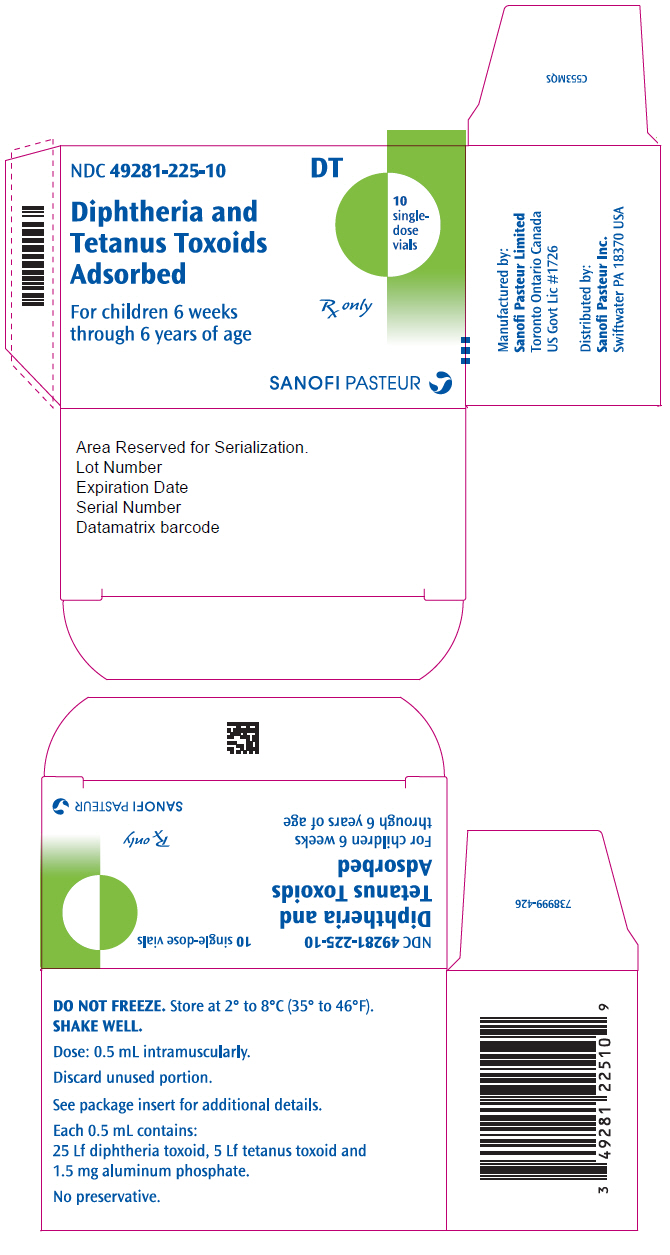
Diphtheria and Tetanus Toxoids Adsorbed is a vaccine indicated for active immunization against diphtheria and tetanus. Diphtheria and Tetanus Toxoids Adsorbed is approved for use in children from 6 weeks through 6 years of age (prior to 7th birthday). (1)
DOSAGE AND ADMINISTRATION
The five dose immunization series consists of an injection administered at 2, 4, 6, 15-18 months and between 4 and 6 years of age. (2.1)
DOSAGE FORMS AND STRENGTHS
Suspension for injection, supplied in single-dose (0.5 mL) vials (3)
CONTRAINDICATIONS
Severe allergic reaction (e.g., anaphylaxis) after a previous dose of Diphtheria and Tetanus Toxoids Adsorbed or any other diphtheria toxoid or tetanus toxoid-containing vaccine, or any other component of this vaccine. (4)
WARNINGS AND PRECAUTIONS
- If Guillain-Barré syndrome occurred within 6 weeks of receipt of prior vaccine containing tetanus toxoid, the risk for Guillain-Barré syndrome may be increased following Diphtheria and Tetanus Toxoids Adsorbed vaccine. (5.2)
- Apnea following intramuscular vaccination has been observed in some infants born prematurely. The decision about when to administer an intramuscular vaccine, including Diphtheria and Tetanus Toxoids Adsorbed, to an infant born prematurely should be based on consideration of the individual infant's medical status and the potential benefits and possible risks of vaccination. (5.5)
- Syncope (fainting) has been reported following vaccination with Diphtheria and Tetanus Toxoids Adsorbed vaccine. Procedures should be in place to prevent falling injury and manage syncopal reactions. (5.6)
ADVERSE REACTIONS
The most common adverse reactions (≥5%) were crying, fever, and loss of appetite. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Sanofi Pasteur Inc., at 1-800-822-2463 (1-800-VACCINE) or VAERS at 1-800-822-7967 and http://vaers.hhs.gov.
DRUG INTERACTIONS
Immunosuppressive therapies may reduce the response to Diphtheria and Tetanus Toxoids Adsorbed. (7.3)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage and Schedule
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
5.2 Guillain-Barré Syndrome and Brachial Neuritis
5.3 Limitation of Vaccine Effectiveness
5.4 Altered Immunocompetence
5.5 Apnea in Premature Infants
5.6 Syncope
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Concomitant Administration with Other Vaccines
7.2 Concomitant Administration with Tetanus Immune Globulin (Human)
7.3 Immunosuppressive Treatments
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Diphtheria and Tetanus Toxoids Adsorbed is a vaccine indicated for active immunization against diphtheria and tetanus. Diphtheria and Tetanus Toxoids Adsorbed is approved for use in children from 6 weeks through 6 years of age (prior to 7th birthday).
Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine (DTaP) or a DTaP-containing vaccine is recommended for immunization of infants and children 6 weeks through 6 years of age. Diphtheria and Tetanus Toxoids Adsorbed should be used in instances where the pertussis vaccine component is contraindicated.
Diphtheria and Tetanus Toxoids Adsorbed is not to be used for treatment of diphtheria or tetanus infection.
-
2 DOSAGE AND ADMINISTRATION
For intramuscular use only.
2.1 Dosage and Schedule
Diphtheria and Tetanus Toxoids Adsorbed is approved for administration as a 5 dose series at 2, 4, 6, 15-18 months, and 4-6 years. The first dose of Diphtheria and Tetanus Toxoids Adsorbed may be administered as early as 6 weeks of age.
2.2 Administration
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If these conditions exist, the product should not be administered.
After removing the "flip-off" cap, cleanse the vaccine vial stopper with a suitable germicide. Do not remove either the rubber stopper or the metal seal holding it in place. Just before use, shake the vial well until a uniform, white, cloudy suspension results.
Using a sterile needle and syringe and aseptic technique, withdraw and administer a single 0.5 mL dose of Diphtheria and Tetanus Toxoids Adsorbed intramuscularly. Use a separate sterile needle and syringe for each injection. Changing needles between withdrawing the vaccine from the vial and injecting it into a recipient is not necessary unless the needle has been damaged or contaminated. In infants younger than 1 year, the anterolateral aspect of the thigh provides the largest muscle and is the preferred site of injection. In older children, the deltoid muscle is usually large enough for injection. The vaccine should not be injected into the gluteal area or areas where there may be a major nerve trunk.
Diphtheria and Tetanus Toxoids Adsorbed vaccine should not be combined through reconstitution or mixed with any other vaccine. Discard unused portion.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
A severe allergic reaction (e.g., anaphylaxis) after a previous dose of Diphtheria and Tetanus Toxoids Adsorbed or any other diphtheria toxoid or tetanus toxoid-containing vaccine, or any other component of this vaccine is a contraindication to administration of Diphtheria and Tetanus Toxoids Adsorbed. [See Description (11).]
-
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
Epinephrine Injection (1:1000) and other appropriate agents and equipment must be available for immediate use in case an anaphylactic or acute hypersensitivity reaction occurs.
5.2 Guillain-Barré Syndrome and Brachial Neuritis
A review by the Institute of Medicine (IOM) found evidence for a causal relation between tetanus toxoid and both brachial neuritis and Guillain-Barré syndrome. (1) If Guillain-Barré syndrome occurred within 6 weeks of receipt of prior vaccine containing tetanus toxoid, the risk for Guillain-Barré syndrome may be increased following Diphtheria and Tetanus Toxoids Adsorbed vaccine.
5.3 Limitation of Vaccine Effectiveness
Vaccination with Diphtheria and Tetanus Toxoids Adsorbed may not protect all individuals.
5.4 Altered Immunocompetence
If Diphtheria and Tetanus Toxoids Adsorbed vaccine is administered to immunocompromised persons, including persons receiving immunosuppressive therapy, the expected immune response may not be obtained. [See Immunosuppressive Treatments (7.3).]
5.5 Apnea in Premature Infants
Apnea following intramuscular vaccination has been observed in some infants born prematurely. The decision about when to administer an intramuscular vaccine, including Diphtheria and Tetanus Toxoids Adsorbed, to an infant born prematurely should be based on consideration of the individual infant's medical status and the potential benefits and possible risks of vaccination.
-
6 ADVERSE REACTIONS
The most common adverse reactions (≥5%) were crying, fever, and loss of appetite.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to vaccine use and for approximating rates of those events.
In a clinical trial in Baltimore, 163 infants received Diphtheria and Tetanus Toxoids Adsorbed at 2, 4 and 6 months of age. The results of this trial are presented in Table 1.
Table 1: Percentage of Children Experiencing Local and Systemic Reactions at 24 Hours Following Immunization Reaction BALTIMORE*
(N=163)Dose 1
(%)
(n = 155)Dose 2
(%)
(n = 145)Dose 3
(%)
(n = 136)- *
- A total of 163 children received one of the three lots of Diphtheria and Tetanus Toxoids Adsorbed at 2, 4, and 6 months of age, and acellular pertussis vaccine at 3, 5, and 7 months of age. One control group (N=85) received Diphtheria and Tetanus Toxoids Adsorbed concurrently at a separate site with acellular pertussis vaccine at 2, 4 and 6 months of age (data not shown). A second control group (N=85) received commercial DTwP vaccine at 2, 4, and 6 months of age, and a placebo at 3, 5, and 7 months of age (data not shown).
Systemic Reactions Fever ≥38°C <39°C (≥100.4°F <102.2°F) 0.7 0.8 6.6 Fever ≥39°C (≥102.2°F ) 0 0 0 Crying 13.6 15.2 13.0 Loss of Appetite 3.9 6.2 2.9 Injection Site Reactions Redness ≥2.5 cm 0.7 0 3.6 Slight Pain 2.6 2.8 2.2 Moderate Pain 0.7 1.4 0 Hardness ≥2.5 cm 1.3 1.4 3.6 Two clinical trials were conducted in Canada. In the first clinical trial, 52 children aged 17-22 months who had previously received 3 doses of whole-cell DTP Adsorbed vaccine (not licensed in US), received Diphtheria and Tetanus Toxoids Adsorbed with either an acellular pertussis (n = 25) or a whole cell pertussis (n = 27) vaccine (neither licensed in US) given concurrently but at a separate site. The only reported local reaction was slight pain at the Diphtheria and Tetanus Toxoids Adsorbed injection site in 11% of children.
In a second clinical trial conducted in Canada, 99 children aged 4 to 6 years old who were eligible for the preschool (fifth) dose of DTP received Diphtheria and Tetanus Toxoids Adsorbed in one arm and a whole-cell Monovalent Pertussis vaccine (not licensed in US) in the other. The following local reactions at the Diphtheria and Tetanus Toxoids Adsorbed injection site were reported: redness ≥50 mm - 9%, swelling >50 mm - 51%, tenderness, moderate or severe - 17%, arm mobility "too sore to move" - 9%. (2)
Diphtheria and Tetanus Toxoids Adsorbed evaluated in clinical trials contained thimerosal.
6.2 Postmarketing Experience
The following adverse events have been spontaneously reported during the postmarketing use of a Diphtheria and Tetanus Toxoids Adsorbed vaccine manufactured by Sanofi Pasteur Limited that contained thimerosal. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure.
The following adverse events were included based on severity, frequency of reporting or the strength of causal association with Diphtheria and Tetanus Toxoids Adsorbed:
Blood and lymphatic system disorders
- Lymphadenopathy
Gastrointestinal disorders
- Nausea
General disorders and administration site conditions
- Injection site inflammation
- Injection site hypersensitivity
- Pain
Nervous system disorders
- Convulsion
- Somnolence
- Syncope
- Headache
Skin and subcutaneous tissue disorders
- Rash
- Urticaria
Vascular disorders
- Pallor
-
7 DRUG INTERACTIONS
7.1 Concomitant Administration with Other Vaccines
No safety and immunogenicity data are available on the concomitant administration of Diphtheria and Tetanus Toxoids Adsorbed with other US licensed vaccines.
7.2 Concomitant Administration with Tetanus Immune Globulin (Human)
If passive protection against tetanus is required, TIG (Human) may be administered according to its prescribing information, concomitantly with Diphtheria and Tetanus Toxoids Adsorbed at a separate site with a separate needle and syringe.
7.3 Immunosuppressive Treatments
Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs and corticosteroids (used in greater than physiologic doses), may reduce the immune response to Diphtheria and Tetanus Toxoids Adsorbed. [See Warnings and Precautions (5.4).]
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Diphtheria and Tetanus Toxoids Adsorbed is not approved for use in individuals 7 years of age and older. Human or animal data are not available to assess vaccine-associated risks in pregnancy.
8.2 Lactation
Diphtheria and Tetanus Toxoids Adsorbed is not approved for use in individuals 7 years of age and older. Human or animal data are not available to assess the impact of Diphtheria and Tetanus Toxoids Adsorbed on milk production, its presence in breast milk, or its effects on the breastfed infant.
-
11 DESCRIPTION
Diphtheria and Tetanus Toxoids Adsorbed is a sterile, cloudy, white, uniform suspension of diphtheria and tetanus toxoids adsorbed on aluminum phosphate and suspended in isotonic sodium chloride solution for intramuscular injection only. Diphtheria and Tetanus Toxoids Adsorbed vaccine does not contain a preservative.
Each 0.5 mL dose is formulated to contain: 25 Lf diphtheria toxoid and 5 Lf tetanus toxoid. Other ingredients per 0.5 mL dose include: 1.5 mg aluminum phosphate and <100 mcg free formaldehyde.
Diphtheria toxoid is prepared from the toxin produced during the growth of a selected strain of Corynebacterium diphtheriae grown with aeration in submerged culture. The toxin is purified by precipitation, converted to toxoid by the addition of formalin and concentrated by ultrafiltration. The culture medium consists of a tryptic digest of casein, supplemented with cystine, maltose, uracil, inorganic salts and vitamins.
Tetanus toxoid is prepared from the toxin produced during the growth of a selected strain of Clostridium tetani. The toxin is converted to toxoid by the addition of formalin, concentrated and then purified. The culture medium consists of a tryptic digest of casein, supplemented with cystine, dextrose, uracil, inorganic salts and vitamins.
When tested in guinea pigs, the tetanus and diphtheria components induce at least 2 neutralizing units/mL of serum.
The vial stopper is not made with natural rubber latex.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Diphtheria is an acute toxin-mediated disease caused by toxigenic strains of C. diphtheriae. Protection against disease is due to the development of neutralizing antibodies to diphtheria toxin. A serum diphtheria antitoxin level of 0.01 International Units (IU)/mL is the lowest level giving some degree of protection, and levels of at least 0.1 IU/mL are generally regarded as protective. (3) (4)
Tetanus is an acute disease caused by an extremely potent neurotoxin produced by C. tetani. Protection against disease is due to the development of neutralizing antibodies to tetanus toxin. A serum tetanus antitoxin level of 0.01 IU/mL, measured by neutralization assay is considered the minimum protective level. (3) (5)
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
In a clinical study conducted in Baltimore, MD, infants received one of three lots of Diphtheria and Tetanus Toxoids Adsorbed (formulation that contained thimerosal), 0.5 mL, at 2, 4 and 6 months of age. Oral poliovirus vaccine (no longer licensed in the US) was administered concomitantly with Diphtheria and Tetanus Toxoids Adsorbed at 2 and 4 months of age. Diphtheria and tetanus antitoxin levels were evaluated at 8 months of age (see Table 2). Protective levels of diphtheria antitoxin (≥0.01 IU/mL) and tetanus antitoxin (≥0.01 IU/mL) were detected in 99% and 100%, respectively, of the Diphtheria and Tetanus Toxoids Adsorbed recipients after 3 doses. The geometric mean titers (GMT's) for diphtheria and tetanus antitoxin antibodies in recipients of the three Diphtheria and Tetanus Toxoids Adsorbed lots were not significantly different, ranging from 0.25 to 0.35 IU/mL for diphtheria antitoxin antibodies, and from 0.75 to 0.80 IU/mL for tetanus antibodies after the third dose. In a fourth group of 75 infants who received an investigational acellular pertussis vaccine simultaneously with the Diphtheria and Tetanus Toxoids Adsorbed but at separate sites with separate needles and syringes, protective diphtheria and tetanus antitoxin levels developed in 100% of the recipients.
Table 2: Percentage of Children Protected Following Administration of Diphtheria and Tetanus Toxoids Adsorbed Post Dose 3 Diphtheria and Tetanus Toxoids Adsorbed Diphtheria antitoxin ≥0.01 IU/mL 99% (135/136) Tetanus antitoxin ≥0.01 IU/mL 100% (137/137) -
15 REFERENCES
- 1
- Adverse Events Associated with Childhood Vaccines. Institute of Medicine. 1994.
- 2
- Scheifele D, et al. Role of whole-cell pertussis vaccine in severe local reactions to the preschool (fifth) dose of diphtheria-pertussis-tetanus vaccine. Can Med Assoc Journal 1994;150(1).
- 3
- Department of Health and Human Services, Food and Drug Administration. Biological products; bacterial vaccines and toxoids; implementation of efficacy review; proposed rule. Federal Register 1985;50(240):51002-117.
- 4
- Vitek CR, Wharton M. Diphtheria toxoid. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5th ed. Philadelphia, PA: W.B. Saunders; 2008. p. 139-56.
- 5
- Wassilak SGF, et al. Tetanus toxoid. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5th ed. Philadelphia, PA: W.B. Saunders; 2008. p. 805-39.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Inform the parent or guardian of the following:
- It is important to complete the immunization series for maximum protection against diphtheria and tetanus.
- Common adverse reactions include local redness, swelling, and tenderness at the injection site, fever, crying, and loss of appetite.
- Other adverse reactions can occur. Call your healthcare provider with any adverse reactions of concern.
- Provide the Vaccine Information Statements (VIS), which are required by the National Childhood Vaccine Injury Act of 1986.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 0.5 mL Vial Label
- PRINCIPAL DISPLAY PANEL - 10 Vial Package
-
INGREDIENTS AND APPEARANCE
DIPHTHERIA AND TETANUS TOXOIDS ADSORBED
corynebacterium diphtheriae toxoid antigen (formaldehyde inactivated) and clostridium tetani toxoid antigen (formaldehyde inactivated) injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:49281-225 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CORYNEBACTERIUM DIPHTHERIAE TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: IRH51QN26H) (CORYNEBACTERIUM DIPHTHERIAE TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:IRH51QN26H) CORYNEBACTERIUM DIPHTHERIAE TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) 25 [Lf] in 0.5 mL CLOSTRIDIUM TETANI TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: K3W1N8YP13) (CLOSTRIDIUM TETANI TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:K3W1N8YP13) CLOSTRIDIUM TETANI TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) 5 [Lf] in 0.5 mL Inactive Ingredients Ingredient Name Strength ALUMINUM PHOSPHATE (UNII: F92V3S521O) 1.5 mg in 0.5 mL FORMALDEHYDE (UNII: 1HG84L3525) 100 ug in 0.5 mL Product Characteristics Color WHITE (CLOUDY) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49281-225-10 10 in 1 PACKAGE 1 NDC:49281-225-58 0.5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103944 03/29/2010 Labeler - Sanofi Pasteur Inc. (086723285) Establishment Name Address ID/FEI Business Operations Sanofi Pasteur Limited 208206623 MANUFACTURE