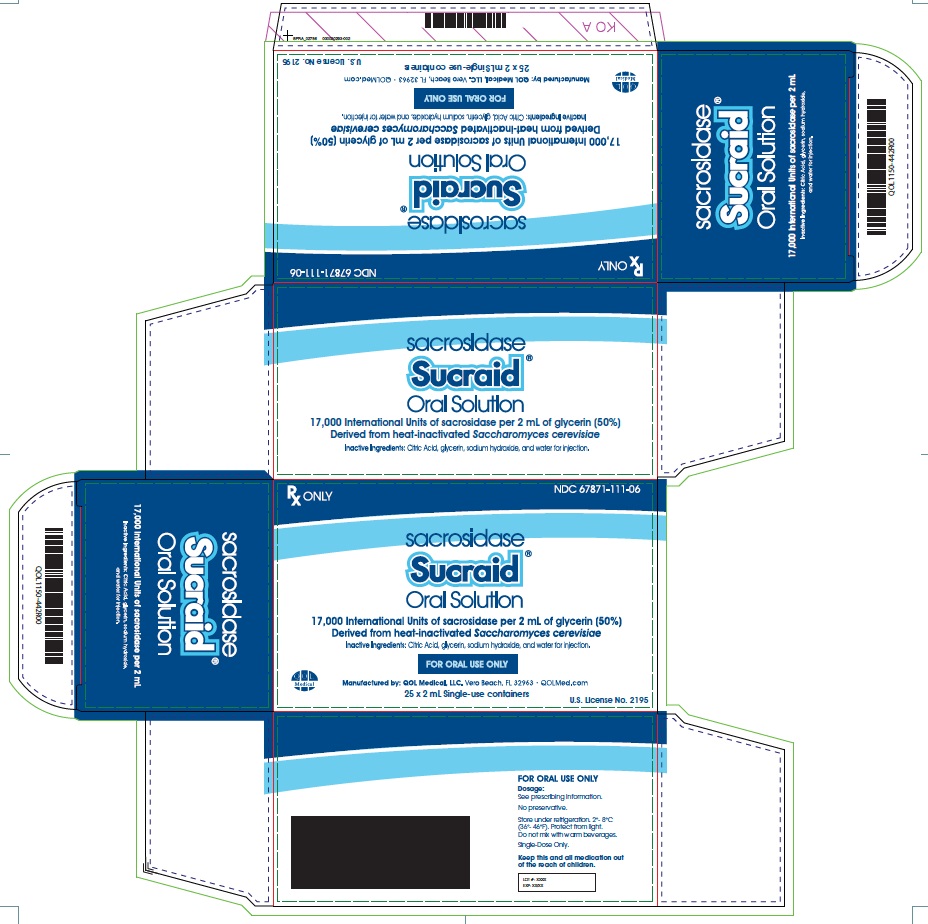
Label: SUCRAID- sacrosidase solution
-
NDC Code(s):
67871-111-01,
67871-111-04,
67871-111-05,
67871-111-06, view more67871-111-07
- Packager: QOL Medical, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated August 7, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Sacrosidase is an enzyme with the chemical name of β,D-fructofuranoside fructohydrolase. The enzyme is derived from baker’s yeast (Saccharomyces cerevisiae). It has been reported that the primary amino acid structure of this protein consists of 513 amino acids with an apparent molecular weight of 100,000 Da for the glycosylated monomer (range 66,000- 116,000 Da). Reports also suggest that the protein exists in solution as a monomer, dimer, tetramer, and octomer ranging from 100,000 Da to 800,000 Da. It has an isoelectric point (pI) of 4.5.
Sucraid® (sacrosidase) Oral Solution is an oral enzyme replacement therapy.
Sucraid is a pale yellow to colorless, clear solution with a pleasant, sweet taste. Each milliliter (mL) of Sucraid contains 8,500 International Units (I.U.) of the enzyme sacrosidase, the active ingredient.
Sucraid may contain small amounts of papain (see WARNINGS). Papain is a protein-cleaving enzyme that is introduced in the manufacturing process to digest the cell wall of the yeast and may not be completely removed during subsequent process steps. Sucraid contains sacrosidase in a vehicle comprised of glycerin, water, citric acid, and sodium hydroxide to maintain the pH at 4.0 to 4.7. Glycerol (glycerin) in the amount consumed in the recommended doses of Sucraid has no expected toxicity.
This enzyme preparation is fully soluble with water, milk, and infant formula. DO NOT HEAT SOLUTIONS CONTAINING SUCRAID. Do not put Sucraid in warm or hot liquids (see DOSAGE AND ADMINISTRATION, Administration Instructions).
-
CLINICAL PHARMACOLOGY
Congenital sucrase-isomaltase deficiency (CSID) is a chronic, autosomal recessive, inherited, phenotypically heterogeneous disease with very variable enzyme activity. CSID is usually characterized by a complete or almost complete lack of endogenous sucrase activity, a very marked reduction in isomaltase activity, and a moderate decrease in maltase activity.
Sucrase is naturally produced in the brush border of the small intestine, primarily the distal duodenum and jejunum. Sucrase hydrolyzes the disaccharide sucrose into its component monosaccharides, glucose and fructose. Isomaltase breaks down disaccharides from starch into simple sugars. Sucraid does not contain isomaltase.
In the absence of endogenous human sucrase, as in CSID, sucrose is not metabolized. Unhydrolyzed sucrose and starch are not absorbed from the intestine and their presence in the intestinal lumen may lead to osmotic retention of water. This may result in loose stools.
Unabsorbed sucrose in the colon is fermented by bacterial flora to produce increased amounts of hydrogen, methane, and water. As a consequence, excessive gas, bloating, abdominal cramps, diarrhea, nausea, and vomiting may occur.
Chronic malabsorption of disaccharides may result in malnutrition. Undiagnosed/untreated CSID patients often fail to thrive and fall behind in their expected growth and development curves. Previously, the treatment of CSID has required the continual use of a strict sucrose-free diet.
-
CLINICAL STUDIES
A two-phase (dose response preceded by a breath hydrogen phase) double-blind, multi-site, crossover trial was conducted in 28 pediatric patients (approximately 5 months to 12 years of age) with confirmed CSID. During the dose response phase, the patients were challenged with an ordinary sucrose-containing diet while receiving each of four doses of sacrosidase: full strength (9000 I.U./mL) and three dilutions (1:10 [900 I.U./mL], 1:100 [90 I.U./mL], and 1:1000 [9 I.U./mL]) in random order for a period of 10 days. Patients who weighed no more than 15 kg received 1 mL per meal; those weighing more than 15 kg received 2 mL per meal. The dose did not vary with age or sucrose intake. A dose-response relationship was shown between the two higher and the two lower doses. The two higher doses of sacrosidase were associated with significantly fewer total stools and higher proportions of patients having lower total symptom scores, the primary efficacy end-points. In addition, higher doses of sacrosidase were associated with a significantly greater number of hard and formed stools as well as with fewer watery and soft stools, the secondary efficacy end-points.
Analysis of the overall symptomatic response as a function of age indicated that in CSID pediatric patients up to 3 years of age, 86% became asymptomatic. In pediatric patients over 3 years of age, 77% became asymptomatic. Thus, the therapeutic response did not differ significantly according to pediatric age.
A second study of similar design and execution as the first used 4 different dilutions of sacrosidase: 1:100 (90 I.U./mL), 1:1000 (9 I.U./mL), 1:10,000 (0.9 I.U./mL), and 1:100,000 (0.09 I.U./mL). There were inconsistent results with regards to the primary efficacy parameters.
In both trials, however, pediatric patients showed a marked decrease in breath hydrogen output when they received sacrosidase in comparison to placebo.
The effects of Sucraid have not been evaluated in patients with secondary (acquired) sucrase deficiency.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Severe Hypersensitivity Reactions
Severe hypersensitivity reactions, including wheezing, rash, and pruritis, have been reported with administration of Sucraid. Sucraid contains papain, which is associated with hypersensitivity reactions (see DESCRIPTION).
A pediatric patient in the clinical trials experienced a hypersensitivity reaction of severe wheezing that required hospitalization. Postmarketing cases of cutaneous hypersensitivity reactions have also been reported.
Instruct patients or caregivers to stop Sucraid and seek medical attention if symptoms suggestive of a hypersensitivity reaction occur. Sucraid is contraindicated in patients who have had a known hypersensitivity reaction (see CONTRAINDICATIONS).
-
PRECAUTIONS
Increased Blood Glucose Concentrations in Patients with Diabetes Mellitus
Sucraid enables the products of sucrose hydrolysis, glucose and fructose, to be absorbed and may increase blood glucose concentrations. Monitor blood glucose concentrations and adjust the diet accordingly for patients with diabetes mellitus.
Dietary Starch Restriction
Sucraid does not replace isomaltase. Therefore, patients may still experience symptoms of CSID while taking Sucraid. Consider dietary starch restriction in addition to Sucraid, especially in patients in whom symptoms are not adequately controlled by Sucraid.
Drug Interactions
Fruit Juice
The acidity in fruit juice may reduce the enzyme activity in Sucraid. Administration of Sucraid with liquids other than water, milk, or infant formula has not been studied and is not recommended (see DOSAGE AND ADMINISTRATION, Administration Instructions).
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals with Sucraid have not been performed to evaluate the carcinogenic potential. Studies to evaluate the effect of Sucraid on fertility or its mutagenic potential have not been performed.
Pregnancy
Teratogenic Effects
Animal reproduction studies have not been conducted with Sucraid. Sucraid is not expected to cause fetal harm when administered to a pregnant woman or to affect reproductive capacity. Sucraid should be given to a pregnant woman only if clearly needed.
Nursing Mothers
The Sucraid enzyme is broken down in the stomach and intestines, and the component amino acids and peptides are then absorbed as nutrients.
Pediatric Use
The safety and effectiveness of Sucraid for the treatment of sucrase deficiency, which is part of congenital sucrase-isomaltase deficiency (CSID), have been established in pediatric patients aged 5 months and older. Use of Sucraid for this indication is supported by evidence from adequate and well-controlled studies in pediatric patients (see CLINICAL STUDIES and ADVERSE REACTIONS).
-
ADVERSE REACTIONS
The following adverse reactions associated with the use of sacrosidase were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
In clinical studies of up to 54 months duration, a total of 52 patients were treated with Sucraid. The reported adverse reactions (number of patients) were as follows: abdominal pain (4), vomiting (3), nausea (2), diarrhea (2), constipation (2), insomnia (1), headache (1), nervousness (1), and dehydration (1).
Hypersensitivity reactions (wheezing, rash, and pruritis) have been reported (see WARNINGS).
-
DOSAGE AND ADMINISTRATION
Important Administration Information
- Administer Sucraid with each meal or snack.
- Mix Sucraid with cold or room temperature water, milk or infant formula prior to administration. Administration of Sucraid in liquids other than water, milk, or infant formula has not been studied and is not recommended. Do not mix or consume Sucraid with fruit juice.
- Do not warm or heat the water, milk, or infant formula before or after addition of Sucraid.
- Administer half of the dose at the beginning of the meal or snack and the other half of the dose during the meal or snack.
Recommended Dosage
The recommended dosage is:- Patients weighing 15 kg and less: 8,500 International Units (1 mL) administered orally with each meal or snack.
- Patients weighing more than 15 kg: 17,000 International Units (2 mL) administered orally with each meal or snack.
Preparation and Administration Instructions for Patients Weighing 15 kg or Less
Multiple-Dose Bottle:- Using the measuring scoop provided, add 1 scoop of Sucraid (1 mL) to 60 mL of cold or room temperature water, milk, or infant formula.
- Stir to mix well.
- Administer half of the mixed Sucraid solution (30 mL) at the beginning of the meal or snack and the other half of the mixed solution (30 mL) during the meal or snack.
- Do not save any of the mixed Sucraid solution for later use.
- Rinse the measuring scoop with water.
Single-Use Container:
- Empty the entire contents of the single-use container (2 mL) in 120 mL of cold or room temperature water, milk, or infant formula.
- Stir to mix well.
- Divide the mixed Sucraid solution into two separate 60 mL portions. The first portion (60 mL) is for immediate use.
• Administer half of the first portion (30 mL) of the mixed Sucraid solution at the beginning of the meal or snack and the other half of the first portion (30 mL) of the mixed Sucraid solution during the meal or snack. - Store the second portion of the mixed Sucraid solution (60 mL) at 2°C to 8°C (36°F to 46°F) for up to 24 hours for administration with the next meal or snack.
• Discard the mixed Sucraid solution if not used within 24 hours.
Preparation and Administration Instructions for Patients Weighing More than 15 kg
Multiple-Dose Bottle:- Using the measuring scoop provided, add 2 scoops of Sucraid (2 mL) to 120 mL of cold or room temperature water, milk, or infant formula.
- Stir to mix well.
- Administer half of the mixed Sucraid solution (60 mL) at the beginning of the meal or snack and the other half of the mixed Sucraid solution (60 mL) during the meal or snack.
- Do not save any of the mixed Sucraid solution for later use.
- Rinse the measuring scoop with water.
Single-Use Container:
- Empty the entire contents of the single-use container (2 mL) in 120 mL of cold or room temperature water, milk, or infant formula.
- Stir to mix well.
- Administer half of the mixed Sucraid solution (60 mL) at the beginning of the meal or snack and the other half of the mixed solution during the meal or snack (60 mL).
- Do not save any of the mixed Sucraid solution for later use.
-
HOW SUPPLIED
118 mL Multiple-Dose Bottle
Sucraid (sacrosidase) Oral Solution is available in 118 mL (4 fluid ounces) multiple-dose translucent plastic bottles, packaged two bottles per carton. Each mL of solution contains 8,500 International Units of sacrosidase. A 1 mL measuring scoop is provided with each bottle. A full measuring scoop is 1 mL.
NDC# 67871-111-04 (2 x 118 mL multiple-dose bottles)
Store under refrigeration at 2°C to 8°C (36°F to 46°F). Discard four weeks after first opening due to the potential for bacterial growth. Protect from heat and light.
2 mL Single-Use Container
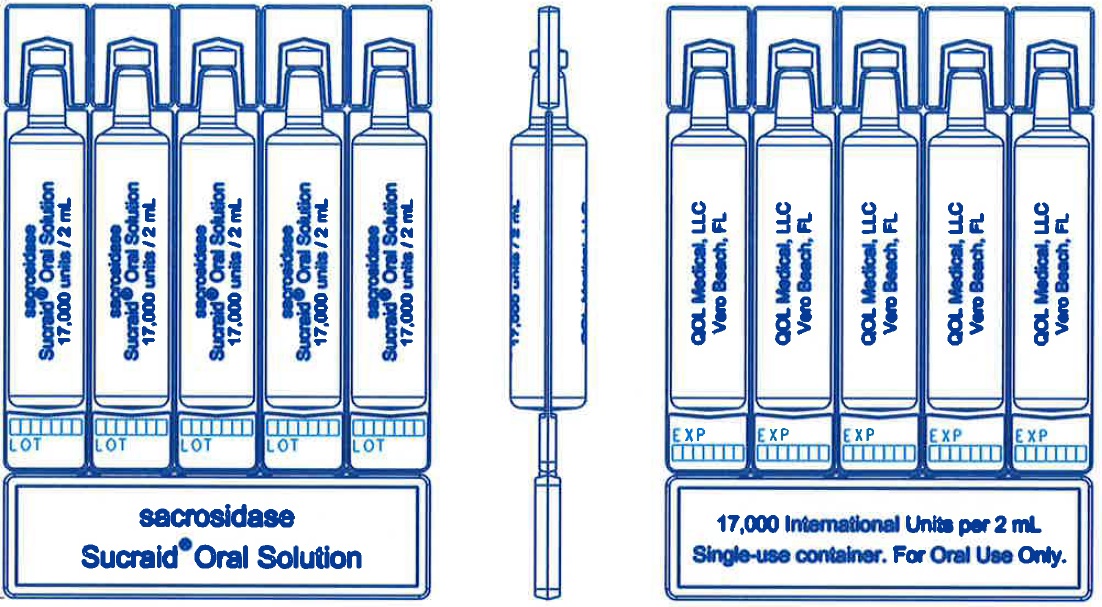
Sucraid (sacrosidase) Oral Solution is available in 2 mL, single-use containers that are packaged into a foil pouch. Each 2 mL single-use container contains 17,000 International Units of sacrosidase. Each foil pouch holds a card of 5 containers. Five pouches are then packaged in a box (25 containers). Six boxes are further packaged in a carton (150 containers).
NDC# 67871-111-07 (150 x 2 mL single-use containers)
Store under refrigeration, 2°C to 8°C (36°F to 46°F). Protect from light. Single-use container can be removed from refrigeration and stored at 15°C to 25°C (59°F to 77°F) for up to 3 days (72 hours).
Manufactured by:
QOL Medical, LLC Vero Beach, FL 32963
U.S. License No. 2195www.sucraid.com
For questions call 1-866-469-3773Rev <08/24>
-
Instructions for Use
Sucraid® (Su-kreid) (sacrosidase) Oral Solution:
2-mL Single-Use Container
Read this Instructions for Use before you start taking or giving Sucraid to a child, and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your or your child’s medical condition or treatment.
Important information you need to know before taking or giving Sucraid:
- The 2-mL single-use container is for children and adults.
- Sucraid is supplied in 2-mL single-use containers in a foil pouch. Each foil pouch holds 5 single-use containers.
Each container is one 2 mL Sucraid dose.
- Your healthcare provider will decide the right dose of Sucraid for you or your child. Do not change the dose of Sucraid without talking to your healthcare provider.
- Sucraid can only be dissolved with cold or room temperature water, milk, or infant formula. Do not put Sucraid in warm or hot liquids. Do not dissolve Sucraid with fruit juice. Do not give or take Sucraid with fruit juice.
- Do not warm or heat the mixed solution before taking or giving Sucraid.
- Sucraid should be taken or given with each meal or snack. Half of the Sucraid dose should be taken at the beginning of each meal or snack. Take or give the remaining Sucraid dose during the meal or snack.
- Do not use the Sucraid single-use container if the seal has been damaged. Contact your pharmacist or healthcare provider if you cannot use the Sucraid single-use container.
Supplies needed to take or give Sucraid:
- 1 Sucraid 2-mL container
- 4 ounces of cold or room temperature water, milk, or infant formula (not included)
- Meal or snack (not included)
- Spoon to mix (not included)
How to take or give Sucraid:
Step 1: Check the expiration date on the Sucraid foil pouch. Do not use Sucraid if it is past the expiration date.
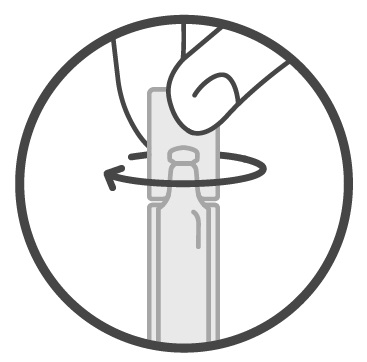
Remove 1 Sucraid 2-mL container from a foil pouch.Step 2: Twist the cap to the left to remove it from the container. See Figure 1.
.
Figure 1
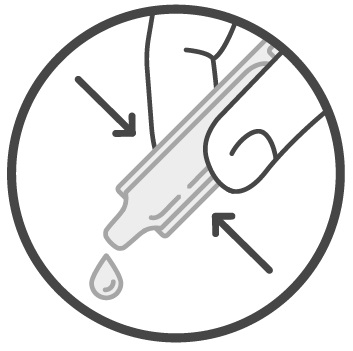
Step 3: Squeeze all the Sucraid solution in the container into 4 ounces of cold or room temperature water, milk, or infant formula. See Figure 2.
Figure 2
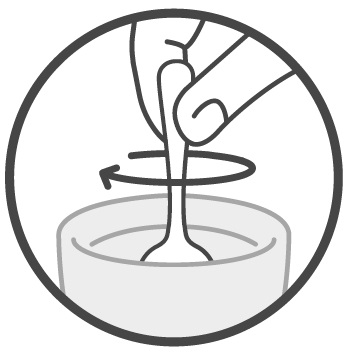
Step 4: Mix your or your child’s prescribed dose in 4 ounces of cold or room temperature water, milk, or infant formula.
See Figure 3.Figure 3
Step 5:
For patients weighing more than 33 pounds (15 kilograms):- The entire 4 ounces of mixed solution will be taken or given during each meal or snack. Take or give half of the mixed solution (2 ounces) at the beginning of the meal or snack and take or give the other half of the mixed solution (2 ounces) during the meal or snack.
For patients weighing 33 pounds (15 kilograms) or less:
- Divide the 4-ounce mixed solution into two separate 2-ounce portions.
- Take or give half of the first portion (1 ounce) at the beginning of the meal or snack and take or give the other half of the first portion (1 ounce) during the meal or snack.
- Store the second portion (2 ounces) in the refrigerator at 36°F to 46°F (2°C to 8°C) for the next meal or snack. Take or give half of the second portion (1 ounce) at the beginning of the next meal or snack and take or give the other half of the second portion (1 ounce) during the meal or snack.
- Throw away the second portion (2 ounces) if you do not use it within 24 hours.
Throwing away (disposal of) Sucraid:
- Throw away expired or empty Sucraid containers in your household trash.
How should I store Sucraid?
• Store the Sucraid single-use container in the refrigerator between 36°F to 46°F (2°C to 8°C).
• The Sucraid single-use container may be stored between 59°F to 77°F (15°C to 25°C) for up to 3 days.
• Protect Sucraid from heat and light.
Keep Sucraid and all medicines out of the reach of children.
Manufactured by:
QOL Medical, LLC Vero Beach, FL 32963
U.S. License No. 2195For more information, go to www.Sucraid.com or call 1-866-469-3773.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: July 2024
-
Instructions for Use
SUCRAID® (Su-kreid)
(sacrosidase)
oral solution
118 mL Multiple-Dose Bottle
Read this Instructions for Use before you start taking or giving SUCRAID to a child, and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your or your child’s medical condition or treatment.

Important information you need to know before taking or giving SUCRAID:
- Your healthcare provider will decide the right dose of SUCRAID for you or your child. Do not change the dose of SUCRAID without talking to your healthcare provider.
- The dose of SUCRAID depends on body weight. Your healthcare provider will tell you how much SUCRAID you should take or give your child.
- The dose for a child 33 pounds (15 kg) or less is 1 mL or 28 drops of SUCRAID in 2 ounces of water, milk, or infant formula.
- The dose for a child or adult more than 33 pounds (15 kg) is 2 mL or 56 drops of SUCRAID in 4 ounces of water, milk, or infant formula.
- SUCRAID can only be dissolved with cold or room temperature water, milk, or infant formula. Do not put SUCRAID in warm or hot liquids. Do not dissolve SUCRAID with fruit juice. Do not take or give SUCRAID with fruit juice.
- Do not warm or heat the mixed solution before taking or giving SUCRAID.
- Measure your dose or your child’s dose of SUCRAID using the measuring scoop that comes with the SUCRAID bottle. Do not use a kitchen teaspoon or other measuring device.
- SUCRAID should be taken or given with each meal or snack. Half of the SUCRAID dose should be taken or given at the beginning of each meal or snack. Take or give the remaining SUCRAID dose during the meal or snack.
- Do not use the SUCRAID multiple-dose bottle if the seal has been damaged. Contact your pharmacist or healthcare provider if you cannot use the SUCRAID multiple-dose bottle.
Supplies needed to take or give SUCRAID:
- SUCRAID 118 mL multiple-dose bottle
- 1 measuring scoop (included in SUCRAID carton)
- 2 to 4 ounces of cold or room temperature water, milk, or infant formula (not included)
- Meal or snack (not included)
How to take or give SUCRAID:
Step 1: Check the expiration date on the SUCRAID bottle. Do not use SUCRAID after the expiration date on the bottle has passed.
Step 2: Write down the date the bottle is first opened in the space provided on the bottle label.
Step 3: Each bottle of SUCRAID has a plastic screw cap that covers a dropper dispensing tip. Remove the plastic screw cap by twisting it to the left.
Step 4: Use the measuring scoop that comes in your SUCRAID carton to measure your or your child’s prescribed dose. See Figure 1. Reseal the bottle after each use by replacing and twisting the plastic screw cap to the right until tight.
Figure 1
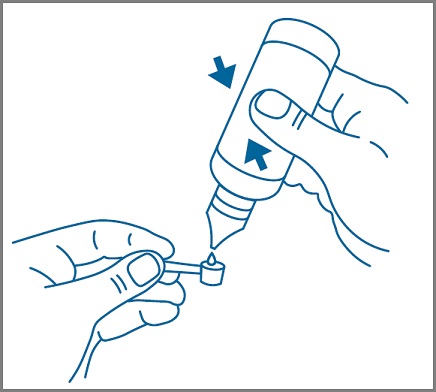
Step 5: Mix your or your child’s prescribed dose in 2 ounces or 4 ounces of cold or room temperature water, milk, or infant formula as instructed by your healthcare provider. See Figure 2.Figure 2
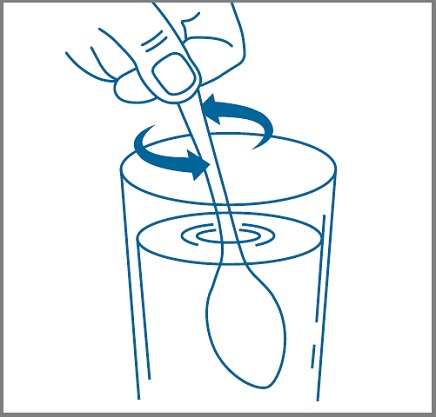
Step 6: Take or give half of the mixed solution at the beginning of each meal or snack. Take or give the remaining mixed solution during the meal or snack.
Step 7: Rinse the measuring scoop with water after each use.
Throwing away (disposal of) SUCRAID:
- Throw away (discard) the SUCRAID multiple-dose bottle and any remaining medicine in your household trash 4 weeks after first opening.
How should I store SUCRAID?
- Store the SUCRAID multiple-dose bottle in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Protect SUCRAID from heat and light.
Keep SUCRAID and all medicines out of the reach of children.
Manufactured by:
QOL Medical, LLC Vero Beach, FL 32963U.S. License No. 2195
For more information, go to www.sucraid.com or call 1-866-469-3773.
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: May 2022
-
INFORMATION FOR PATIENTS
Patient Information
SUCRAID® (Su-kreid)
(sacrosidase)
Oral SolutionWhat is SUCRAID?
SUCRAID is a prescription medicine for the treatment of people who were born with a lack of (deficiency) sucrase, which is part of congenital sucrase-isomaltase deficiency (CSID). It is not known if SUCRAID is safe and effective in children under 5 months of age.Do not take or give your child SUCRAID if you or your child:
- are allergic to yeast, yeast products, glycerin (glycerol), or papain. See the end of this Patient Information leaflet for a complete list of ingredients in SUCRAID.
Before you take or give your child SUCRAID, tell your healthcare provider about all of your medical conditions, including if you or your child:
- have diabetes. SUCRAID can interact with the food in your diet and may change your blood sugar levels. Your healthcare provider will tell you if your diet or diabetes medicines need to be changed.
- are pregnant or plan to become pregnant. It is not known if SUCRAID will harm your unborn baby.
- are breastfeeding or plan to breastfeed. You and your healthcare provider should decide if you will take SUCRAID while breastfeeding.
How should I take or give SUCRAID?
- See the detailed Instructions for Use that come with this Patient Information leaflet for instructions about the right way to take or give SUCRAID.
- SUCRAID should be taken or given exactly as prescribed by your healthcare provider. Do not change the dose of SUCRAID without talking to your healthcare provider.
- SUCRAID comes in a 118-mL multiple-dose bottle or a 2-mL single-use container. Your healthcare provider will decide which type of SUCRAID is best for you to use.
- The dose of SUCRAID depends on body weight. Your healthcare provider will tell you how much SUCRAID you should take or give your child.
- The dose for a child 33 pounds (15 kg) or less is 1 mL or 28 drops of SUCRAID in 2 ounces of water, milk, or infant formula.
- The dose for a child or adult more than 33 pounds (15 kg) is 2 mL or 56 drops of SUCRAID in 4 ounces of water, milk, or infant formula.
- SUCRAID can only be dissolved in cold or room temperature water, milk, or infant formula. Do not put SUCRAID in warm or hot liquids.
- Do not mix SUCRAID with fruit juice. Do not take or give SUCRAID with fruit juice.
- Do not warm or heat the mixed solution before taking or giving SUCRAID.
- Measure your dose or your child’s dose of SUCRAID using the measuring scoop that comes with the SUCRAID bottle. Do not use a kitchen teaspoon or other measuring device.
- SUCRAID should be taken or given with each meal or snack. Half of the SUCRAID dose should be taken at the beginning of each meal or snack. Take or give the remaining SUCRAID dose during the meal or snack.
- Rinse the measuring scoop with water after each use.
- SUCRAID does not break down some sugars found in foods that have starch, such as wheat, rice, and potatoes. Your healthcare provider may tell you to avoid eating foods with starch.
What are the possible side effects of SUCRAID?
SUCRAID may cause serious side effects, including:
-
severe allergic reactions. Severe allergic reactions have happened in some people taking SUCRAID. Tell your healthcare provider right away or go to the nearest emergency room if you have any of the following symptoms:
- difficulty breathing
- wheezing
- rash
- swelling of the face, lips, mouth, or tongue
Your healthcare provider may need to monitor you or your child carefully when first starting treatment with SUCRAID.
The most common side effects of SUCRAID include:
- stomach (abdominal) pain
- vomiting
- nausea
- diarrhea
- constipation
- problems sleeping
- headache
- nervousness
- dehydration
How should I store SUCRAID?
-
SUCRAID 118 mL multiple-dose bottle
- Store in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Throw away after 4 weeks of first opening the multiple-dose bottle.
- Protect from heat and light.
-
SUCRAID 2-mL single-use container
- Store in the refrigerator between 36°F to 46°F (2°C to 8°C)
- After removing from the refrigerator, the 2-mL single-use container can be stored between 59°F to 77°F (15°C to 25°C) for up to 3 days (72 hours).
- Protect from heat and light.
- Keep SUCRAID and all medicines out of the reach of children.
General information about the safe and effective use of SUCRAID.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use SUCRAID for a condition for which it was not prescribed. Do not give SUCRAID to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about SUCRAID that is written for health professionals.What are the ingredients in SUCRAID?
Active ingredient: sacrosidase
Inactive ingredients: Citric acid, glycerol, sodium hydroxide, and water.
Manufactured by:
QOL Medical, LLC Vero Beach, FL 32963
U.S. License No. 2195
For more information, go to www.Sucraid.com or call 1-866-469-3773.This Patient Package Insert has been approved by the U.S. Food and Drug Administration Revised: August 2024
- 118 mL Multi-dose bottle
- PRINCIPAL DISPLAY PANEL
- 2 mL Single-use container
-
INGREDIENTS AND APPEARANCE
SUCRAID
sacrosidase solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:67871-111 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SACROSIDASE (UNII: 8A7F670F2Y) (SACROSIDASE - UNII:8A7F670F2Y) SACROSIDASE 8500 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67871-111-04 2 in 1 CARTON 04/09/1998 1 NDC:67871-111-01 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:67871-111-07 6 in 1 CARTON 05/25/2022 2 NDC:67871-111-06 25 in 1 BOX 2 NDC:67871-111-05 5 in 1 POUCH 2 2 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA020772 04/09/1998 Labeler - QOL Medical, LLC (140026258) Establishment Name Address ID/FEI Business Operations QOL MEDICAL 104085889 api manufacture(67871-111) , analysis(67871-111) Establishment Name Address ID/FEI Business Operations Lyophilization Services of New England, Inc. (LSNE) 028180765 manufacture(67871-111) Establishment Name Address ID/FEI Business Operations Woodstock Sterile Solutions, Inc. 117895702 manufacture(67871-111)