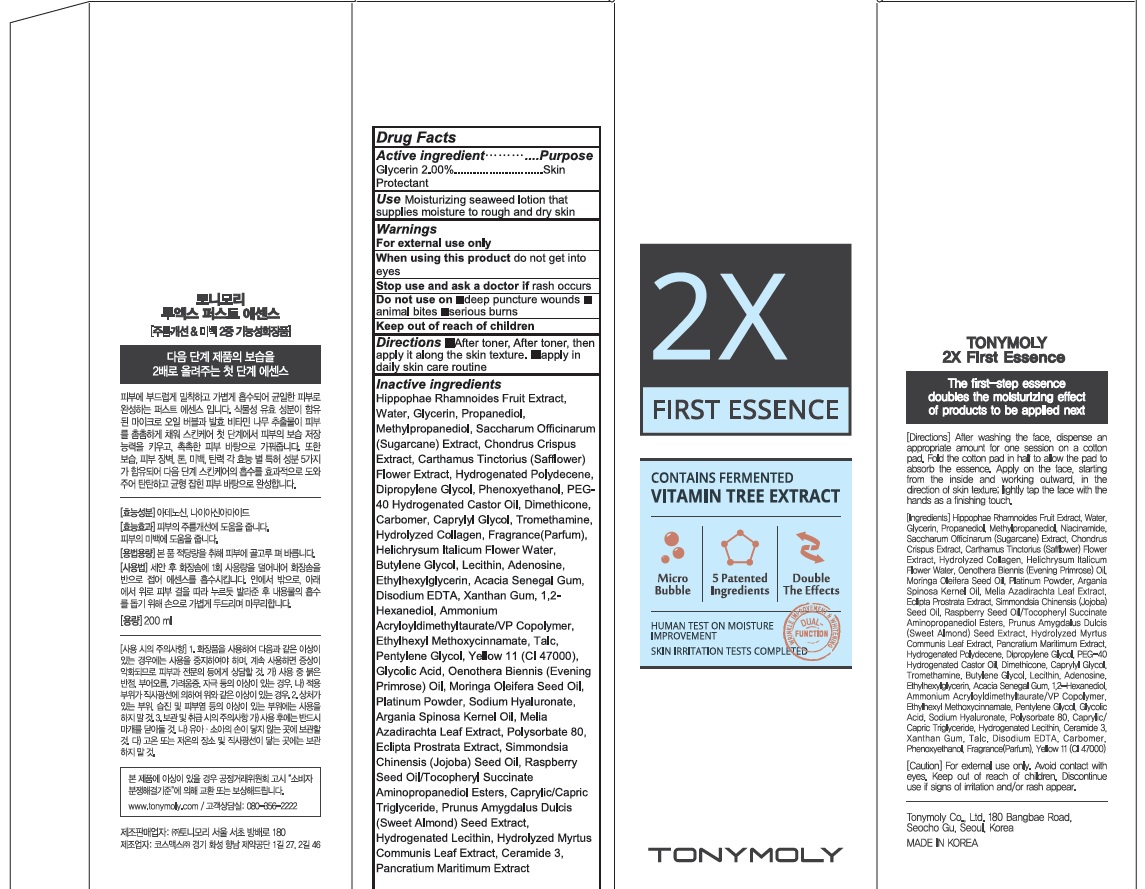
Label: 2X FIRST ESSENCE- glycerin cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 59078-311-01, 59078-311-02 - Packager: TONYMOLY CO.,LTD
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 15, 2016
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Inactive Ingredient: Hippophae Rhamnoides Fruit Extract, Water, Propanediol, Methylpropanediol, Saccharum Officinarum (Sugarcane) Extract, Chondrus Crispus Extract, Carthamus Tinctorius (Safflower) Flower Extract, Hydrogenated Polydecene, Dipropylene Glycol, Phenoxyethanol, PEG-40 Hydrogenated Castor Oil, Dimethicone, Carbomer, Caprylyl Glycol, Tromethamine, Hydrolyzed Collagen, Fragrance(Parfum), Helichrysum Italicum Flower Water, Butylene Glycol, Lecithin, Adenosine, Ethylhexylglycerin, Acacia Senegal Gum, Disodium EDTA, Xanthan Gum, 1,2-Hexanediol, Ammonium Acryloyldimethyltaurate/VP Copolymer, Ethylhexyl Methoxycinnamate, Talc, Pentylene Glycol, Yellow 11 (CI 47000), Glycolic Acid, Oenothera Biennis (Evening Primrose) Oil, Moringa Oleifera Seed Oil, Platinum Powder, Sodium Hyaluronate, Argania Spinosa Kernel Oil, Melia Azadirachta Leaf Extract, Polysorbate 80, Eclipta Prostrata Extract, Simmondsia Chinensis (Jojoba) Seed Oil, Raspberry Seed Oil/Tocopheryl Succinate Aminopropanediol Esters, Caprylic/Capric Triglyceride, Prunus Amygdalus Dulcis (Sweet Almond) Seed Extract, Hydrogenated Lecithin, Hydrolyzed Myrtus Communis Leaf Extract, Ceramide 3, Pancratium Maritimum Extract
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- Use
- Directions
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
2X FIRST ESSENCE
glycerin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59078-311 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Glycerin (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) Glycerin 4.0 g in 200 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Propanediol (UNII: 5965N8W85T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59078-311-02 1 in 1 CARTON 05/01/2016 1 NDC:59078-311-01 200 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/01/2016 Labeler - TONYMOLY CO.,LTD (688216798) Registrant - TONYMOLY CO.,LTD (688216798) Establishment Name Address ID/FEI Business Operations TONYMOLY CO.,LTD 688216798 manufacture(59078-311)