Label: HAEGARDA C1 ESTERASE INHIBITOR SUBCUTANEOUS (HUMAN)- human c1-esterase inhibitor kit
-
NDC Code(s):
63833-765-18,
63833-765-19,
63833-828-02,
63833-829-02, view more63833-838-01, 63833-839-01
- Packager: CSL Behring GmbH
- Category: PLASMA DERIVATIVE
Drug Label Information
Updated February 4, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use HAEGARDA safely and effectively. See full prescribing information for HAEGARDA.
HAEGARDA® (C1 Esterase Inhibitor Subcutaneous [Human])
For Subcutaneous Injection, Freeze-Dried Powder for Reconstitution
Initial U.S. Approval: 2017RECENT MAJOR CHANGES
Indications and Usage (1) 09/2020 INDICATIONS AND USAGE
HAEGARDA is a plasma-derived concentrate of C1 Esterase Inhibitor (Human) (C1-INH) indicated for routine prophylaxis to prevent Hereditary Angioedema (HAE) attacks in patients 6 years of age and older. (1)
DOSAGE AND ADMINISTRATION
For subcutaneous use after reconstitution only.
- Administer 60 International Units per kg body weight twice weekly (every 3 or 4 days). (2)
- Reconstitute HAEGARDA prior to use using Sterile Water for Injection, USP. (2.1)
- Use a silicone-free syringe for reconstitution and administration. (2.1)
- Administer at room temperature within 8 hours after reconstitution. (2.1)
DOSAGE FORMS AND STRENGTHS
HAEGARDA is available as a white lyophilized powder supplied in single-dose vials containing 2000 or 3000 International Units (IU) of C1-INH. (3)
CONTRAINDICATIONS
Do not use in patients with a history of life-threatening immediate hypersensitivity reactions, including anaphylaxis to C1-INH preparations or its excipients. (4)
WARNINGS AND PRECAUTIONS
- Severe hypersensitivity reactions may occur. In case of severe hypersensitivity, discontinue HAEGARDA administration and institute appropriate treatment. Epinephrine should be immediately available for treatment of severe hypersensitivity reaction. (5.1)
- At the recommended subcutaneous (S.C.) dose, a causal relationship between thromboembolic events (TEEs) and the use of HAEGARDA has not been established. However, thrombosis has occurred in treatment attempts with high doses of C1-INH intravenous (I.V.) for prevention or therapy of capillary leak syndrome before, during or after cardiac surgery (unapproved indication and dose). (5.2)
- Because HAEGARDA is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. (5.3)
ADVERSE REACTIONS
- Adverse reactions occurring in more than 4% of subjects treated with HAEGARDA were injection site reactions, hypersensitivity, nasopharyngitis and dizziness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact the CSL Behring Pharmacovigilance Department at 1-866-915-6958 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Preparation and Handling
2.2 Reconstitution and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
5.2 Thromboembolic Events
5.3 Transmissible Infectious Agents
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
After reconstitution, for subcutaneous use only.
HAEGARDA is intended for self (or caregiver)-administration after reconstitution at a dose of 60 International Units (IU) per kg body weight by subcutaneous (S.C.) injection twice weekly (every 3 or 4 days). The patient or caregiver should be trained on how to administer HAEGARDA.
HAEGARDA is provided as a freeze-dried powder for reconstitution with Sterile Water for Injection, USP.
2.1 Preparation and Handling
- Check the expiration date on the product vial label. Do not use beyond the expiration date.
- Work on a clean surface and wash hands before performing the following procedures.
- Prepare and administer using aseptic techniques [see Dosage and Administration (2.2)].
- Use a silicone-free syringe for reconstitution and administration.
- Each vial of HAEGARDA is for single-dose only. Promptly use the reconstituted solution. The solution must be used within 8 hours. Discard partially used vials. HAEGARDA contains no preservative.
- Do not freeze the reconstituted solution.
2.2 Reconstitution and Administration
Use either the Mix2Vial® transfer set provided with HAEGARDA or a commercially available double-ended needle and vented filter spike [see How Supplied/Storage and Handling (16)].
Reconstitution
The procedures below are provided as general guidelines for the reconstitution and administration of HAEGARDA.
HAEGARDA Reconstitution Instructions
- 1.
- Ensure that the HAEGARDA vial and Sterile Water for Injection (diluent) vial are at room temperature.
- 2.
- Place the HAEGARDA vial, diluent vial and Mix2Vial transfer set on a flat surface.
- 3.
- Remove flip caps from the HAEGARDA and diluent vials.
- 4.
- Wipe the stoppers with an alcohol swab and allow to dry prior to opening the Mix2Vial transfer set package.
- 5.
- Open the Mix2Vial transfer set package by peeling away the lid (Figure 1). Do not remove the device from the package.
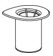
Figure 1 - 6.
- Place the diluent vial on a flat surface and hold the vial tightly. Grip the Mix2Vial transfer set together with the clear package and push the plastic spike at the blue end of the Mix2Vial transfer set firmly through the center of the stopper of the diluent vial (Figure 2).
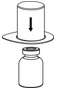
Figure 2 - 7.
- Carefully remove the clear package from the Mix2Vial transfer set. Do not remove the Mix2Vial transfer set or touch the exposed end of the device (Figure 3).
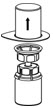
Figure 3 - 8.
- With the HAEGARDA vial placed firmly on a flat surface, invert the diluent vial with the Mix2Vial transfer set attached and push the plastic spike of the transparent adapter firmly through the center of the stopper of the HAEGARDA vial (Figure 4). The diluent will automatically transfer into the HAEGARDA vial.
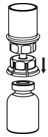
Figure 4 - 9.
- With the diluent and HAEGARDA vial still attached to the Mix2Vial transfer set, gently swirl the HAEGARDA vial to ensure that the powder is fully dissolved (Figure 5). Do not shake the vial.
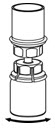
Figure 5 - 10.
- With one hand, grasp the HAEGARDA side of the Mix2Vial transfer set and with the other hand grasp the blue diluent side of the Mix2Vial transfer set, and unscrew the set into two pieces (Figure 6).
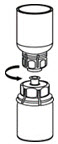
Figure 6 - 11.
- Draw air into an empty, sterile syringe. Use a silicone-free syringe. While the HAEGARDA vial is upright, screw the syringe to the Mix2Vial transfer set. Inject air into the HAEGARDA vial.
- 12.
- While keeping the syringe plunger pressed, invert the system upside down and draw the concentrate into the syringe by pulling the plunger back slowly (Figure 7).
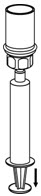
Figure 7 - 13.
- Disconnect the filled syringe by unscrewing it from the Mix2Vial transfer set (Figure 8). The reconstituted solution should be colorless, clear, and free from visible particles. Do not use if particles or discoloration are observed.
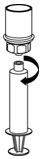
Figure 8 - 14.
- Use immediately or within 8 hours of reconstitution. Store reconstituted solution at room temperature. Do not refrigerate.
- 15.
- If the dose requires more than one vial, use a separate, unused Mix2Vial transfer set and diluent vial for each product vial. Repeat steps 10-12 to pool the contents of the vials into one syringe.
Administration
For subcutaneous injection only.
- Train the patient or caregiver on how to self-administer HAEGARDA.
- Do not mix HAEGARDA with other medicinal products.
- Visually inspect the final solution for particles and discoloration prior to administration, and whenever solution and container permit. Do not use if particles or discoloration is observed.
- Attach the syringe containing the reconstituted HAEGARDA solution to a hypodermic needle or subcutaneous infusion set and administer by subcutaneous injection. Adapt the rate of administration to the comfort level of the patient.
- Inject in the abdominal area or other subcutaneous injection sites. Rotate injection sites so that the same site is not used repeatedly.
- Administer HAEGARDA at room temperature and within 8 hours after reconstitution. Following administration, discard any unused solution and all administration equipment in an appropriate manner as per local requirements.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
HAEGARDA is contraindicated in individuals who have experienced life-threatening hypersensitivity reactions, including anaphylaxis, to C1-INH preparations or its excipients [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS
The physician should discuss the risks and benefits of this product with the patient before prescribing or administering it to the patient [see Patient Counseling Information (17)].
Initiate individualized treatment in case of an acute HAE attack.
5.1 Hypersensitivity
Severe hypersensitivity reactions may occur. The signs and symptoms of hypersensitivity reactions may include hives (local and generalized), tightness of the chest, difficulty breathing, wheezing, hypotension, and/or anaphylaxis during or after injection of HAEGARDA. In case of severe hypersensitivity, discontinue HAEGARDA administration and institute appropriate treatment. Epinephrine should be immediately available for treatment of severe hypersensitivity reaction [see Patient Counseling Information (17)].
5.2 Thromboembolic Events
At the recommended subcutaneous dose, a causal relationship between thromboembolic events (TEEs) and the use of HAEGARDA has not been established [see Patient Counseling Information (17)]. Thrombosis has occurred in treatment attempts with high doses of C1-INH intravenous (I.V.) for prevention or therapy of capillary leak syndrome before, during or after cardiac surgery (unapproved indication and dose).
5.3 Transmissible Infectious Agents
Because HAEGARDA is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. The risk that such products will transmit an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by processes demonstrated to inactivate and/or remove certain viruses during manufacturing [see Description (11) and Patient Counseling Information (17)]. Despite these measures, such products may still contain human pathogenic agents, including those not yet known or identified. Thus, the risk of transmission of infectious agents cannot be totally eliminated.
All infections thought by a physician possibly to have been transmitted by HAEGARDA should be reported by lot number, by the physician or other healthcare provider, to the CSL Behring Pharmacovigilance Department at 1-866-915-6958.
-
6 ADVERSE REACTIONS
Adverse reactions occurring in more than 4% of subjects treated with HAEGARDA were injection site reactions, hypersensitivity, nasopharyngitis and dizziness.
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Of the 90 subjects randomized in the double-blind, placebo-controlled, cross-over study (Study 1) [see Clinical Studies (14)], 86 subjects received at least one dose of HAEGARDA and 86 subjects received at least one dose of placebo (Table 1). A total of 5081 injections of HAEGARDA and placebo were administered over a range of 3 to 19 weeks (median of 16.6 weeks for HAEGARDA; median of 16.3 weeks for placebo). Eligible patients were also able to participate in a randomized, open-label, active treatment-controlled study (Study 2) for up to 140 weeks (n=120).
Table 1. Adverse Reactions in >4% of Subjects Treated with HAEGARDA MedDRA System Organ Class Adverse Reaction HAEGARDA Placebo
(N=86)60 IU/kg
(N=43)40 IU/kg
(N=43)
Overall*
(N=86)n (%) n (%) n (%) n (%) N = number of subjects receiving the treatment; n = number of subjects experiencing ≥1 event. - *
- Includes subjects who were treated with 40 IU/kg or 60 IU/kg HAEGARDA.
- †
- Includes: Injection site bruising, coldness, discharge, erythema, hematoma, hemorrhage, induration, edema, pain, pruritus, rash, reaction, scar, swelling, urticaria, warmth.
- ‡
- Includes: hypersensitivity, pruritus, rash, and urticaria.
General Disorders and Administration Site Conditions Injection Site Reaction† 15
(35)12
(28)27
(31)21
(24)Immune System Disorders Hypersensitivity‡ 3
(7)2
(5)5
(6)1
(1)Infections and Infestations Nasopharyngitis 8
(19)1
(2)9
(11)6
(7)Nervous System Disorders Dizziness 0
(0)4
(9)4
(5)1
(1)Of the injection site reactions occurring after treatment with HAEGARDA, 95% were of mild intensity and 83% resolved within 1 day after onset.
Overall, safety data from the open-label study (Study 2), consisting of 59 patients who participated in Study 1 and 61 patients who did not participate in Study 1 (n=120), were consistent with the safety data from the randomized, double-blind, placebo-controlled, crossover, routine prophylaxis trial (Study 1).
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no prospective clinical data from HAEGARDA use in pregnant women. C1-INH is a normal component of human plasma. Animal developmental or reproduction toxicity studies have not been conducted with HAEGARDA. In the U.S. general population, the estimated background risk of major birth defects occurs in 2-4% of the general population and miscarriage occurs in 15-20% of clinically recognized pregnancies.
Data
In a retrospective case collection study, 22 pregnant women with type I HAE and ranging in age from 20 to 38 years received C1-INH doses of 500 or 1000 IU per I.V. administration for the treatment of acute attacks before, during, and/or after pregnancy (total of 35 pregnancies). No adverse events were associated with C1-INH treatment before, during, or after pregnancy.1
In an observational registry (overall 318 subjects) data were collected on 11 pregnancies in 10 subjects (16 to 40 years old) receiving up to 3000 IU C1-INH (I.V. administration) to treat or prevent HAE attacks. No adverse events were associated with C1-INH treatment.2
In the randomized, open-label, active treatment-controlled, study (Study 2), four pregnant women with type I HAE and ranging in age from 19 to 32 years received C1-INH (S.C. administration). Patients received 40-60 IU/kg per S.C. administration for 4 – 8 weeks (9 - 15 doses) during the first trimester. These women reported no complications during delivery and all women delivered healthy babies.
8.2 Lactation
Risk Summary
There is no information regarding the excretion of HAEGARDA in human milk, the effect on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for HAEGARDA and any potential adverse effects on the breastfed infant from HAEGARDA or from the underlying maternal condition.
Data
In a retrospective case collection study, breastfeeding was documented for neonates from 21 of 35 births with a median duration of 4.8 months (ranging from 1 to 34 months). Mothers were treated postpartum with C1-INH doses up to 1000 IU per I.V. administration for the treatment of acute HAE attacks. No adverse events to the mothers were associated with C1-INH treatment after pregnancy. No information regarding the effect on the breastfed infant was reported.1
8.4 Pediatric Use
The safety and effectiveness of HAEGARDA were evaluated in a subgroup of nine patients 8 to <17 years of age, in the randomized, double-blind, placebo-controlled, crossover, routine prophylaxis trial (Study 1) and the randomized, open-label, active treatment-controlled study (Study 2). Results of subgroup analysis by age were consistent with overall study results.
8.5 Geriatric Use
The safety and effectiveness of HAEGARDA were evaluated in a subgroup of nine subjects 65 to 72 years of age, eight subjects who received the high 60 IU/kg dose and one subject who received the 40 IU/kg dose, in the randomized, double-blind, placebo-controlled, crossover, routine prophylaxis trial (Study 1) and in the randomized, open-label, active treatment-controlled study (Study 2). Clinical studies of HAEGARDA did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
- 10 OVERDOSAGE
-
11 DESCRIPTION
HAEGARDA is a human plasma-derived, purified, pasteurized, lyophilized concentrate of C1-INH to be reconstituted for S.C. administration. HAEGARDA is prepared from large pools of human plasma from U.S. donors. The potency of C1-INH is expressed in International Units (IU), which is related to the current WHO Standard for C1-INH products.
Reconstituted HAEGARDA has a concentration of 500 IU/mL C1-INH, 65 mg/mL total protein, 10 mg/mL glycine, 8.5 mg/mL sodium chloride and 2.7 mg/mL sodium citrate.
C1 Esterase Inhibitor
C1-INH is a soluble, single-chain highly glycosylated protein containing 478 amino acid residues which belongs to the serine protease inhibitor (serpin) family.
All plasma used in the manufacturing of C1-INH is obtained from U.S. donors and is tested using serological assays for hepatitis B surface antigen and antibodies to HIV-1/2 and HCV. Additionally, the plasma is tested with Nucleic Acid Testing (NAT) for HBV, HCV, HIV-1 and HAV and found to be non-reactive (negative). The plasma is also tested by NAT for Human Parvovirus B19. Only plasma that has passed virus screening is used for production, and the limit for Parvovirus B19 in the fractionation pool is set not to exceed 104 IU of Parvovirus B19 DNA per mL.
The manufacturing process for HAEGARDA includes multiple steps that reduce the risk of virus transmission. The virus inactivation/reduction capacity consists of three steps:
- Pasteurization in aqueous solution at 60°C for 10 hours
- Hydrophobic interaction chromatography
- Virus filtration (also called nanofiltration) by two filters, 20 nm and 15 nm, in series.
Viral inactivation and reduction were evaluated in a series of in vitro spiking experiments. The total mean cumulative virus inactivation/reduction is shown in Table 2.
Table 2. Mean Virus Inactivation/Reductions in HAEGARDA Virus Studied Pasteurization
[log10]Hydrophobic Interaction Chromatography
[log10]Virus Filtration
[log10]Total Cumulative [log10] HIV-1, Human immunodeficiency virus type 1, a model for HIV-1 and HIV-2 BVDV, Bovine viral diarrhea virus, a model for HCV PRV, Pseudorabies virus, a model for large enveloped DNA viruses WNV, West Nile virus HAV, Hepatitis A virus CPV, Canine parvovirus B19V, Human Parvovirus B19ND, Not determined NA, Not applicable Enveloped Viruses HIV-1 ≥6.6 ≥4.5 ≥5.1 ≥16.2 BVDV ≥9.2 ≥4.7 ≥5.3 ≥19.2 PRV 6.3 ≥6.5 ≥7.1 ≥19.9 WNV ≥7.0 ND ≥8.0 ≥15.0 Non-Enveloped Viruses HAV ≥6.4 2.8 ≥5.3 ≥14.5 CPV 1.4 3.9 7.1 12.4 B19V 3.9 ND ND NA -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
C1-INH is a normal constituent of human plasma and belongs to the group of serine protease inhibitors (serpins) that includes antithrombin III, alpha1-protease inhibitor, alpha2-antiplasmin, and heparin cofactor II. As with the other inhibitors in this group, C1-INH has an important inhibiting potential on several of the major human cascade systems, including the complement, fibrinolytic and coagulation systems. Regulation of these systems is performed through the formation of complexes between the protease and the inhibitor, resulting in inactivation of both and consumption of the C1-INH.
C1-INH, which is usually activated during the inflammatory process, inactivates its substrate by covalently binding to the reactive site. C1-INH is the only known inhibitor for the C1r and C1s subcomponents of complement component 1 (C1), coagulation factor XIIa, and plasma kallikrein. Additionally, C1-INH is the main inhibitor for coagulation factor XIa of the intrinsic coagulation cascade.
HAE patients have absence or low levels of endogenous or functional C1-INH. Although the events that cause attacks of angioedema in HAE patients are not well defined, it has been postulated that increased vascular permeability and the clinical manifestation of HAE attacks may be primarily mediated through contact system activation. Suppression of contact system activation by C1-INH through the inactivation of plasma kallikrein and factor XIIa is thought to modulate this vascular permeability by preventing the generation of bradykinin. Administration of HAEGARDA replaces the missing or malfunctioning C1-INH protein in patients with HAE.
12.2 Pharmacodynamics
In untreated patients, insufficient levels of functional C1-INH lead to increased activation of C1, which results in decreased levels of complement component 4 (C4). The administration of HAEGARDA increases plasma levels of C1-INH in a dose-dependent manner and subsequently increases plasma concentrations of C4. The C4 plasma concentrations after S.C. administration of 60 IU/kg HAEGARDA were in the normal range (16 to 38 mg/dL).
12.3 Pharmacokinetics
The pharmacokinetics (PK) of C1-INH were described using population PK analysis.
The PK parameters of C1-INH following twice weekly subcutaneous 60 IU/kg dosing are shown in Table 3.
Table 3. Pharmacokinetic Parameter for HAEGARDA (60 IU/kg) from Population Pharmacokinetic Analysis Parameter Mean 95% CI CL (mL/hr/kg)* 1.03 0.90-1.17 Vd (L/kg)* 0.05 0.04-0.06 Bioavailability % 42.7 35.2-50.2 Cmax % 60.7† 31.8-128‡ Ctrough % 48.0† 25.1-102‡ Tmax (hr) 59§ 23-134‡ Half-life (hr)¶ 69§ 24-251‡ The steady state PK of S.C. C1-INH is independent of dose between 20-80 IU/kg in HAE subjects.
Studies have not been conducted to evaluate the PK of C1-INH in specific patient populations stratified by gender, race, or the presence of renal or hepatic impairment. Body weight was included as covariate in the population PK analysis of C1-INH in the age range of 8-72 years while age was not a statistically significant covariate. The body weight adjusted clearance is 11% and 10 % higher in children (8 to < 12 years old) and adolescents (12 to < 18 years old) as compared to adult subjects (18 to 72 years), respectively.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The efficacy and safety of HAEGARDA for routine prophylaxis to prevent HAE attacks were demonstrated in a multicenter, randomized, double-blind, placebo-controlled, crossover study (Study 1), and in a multicenter, randomized, open-label, active treatment-controlled study (Study 2).
Study 1:
The study assessed 90 adult and adolescent subjects with symptomatic HAE type I or II. The median (range) age of subjects was 40 (12 to 72) years; 60 subjects were female and 30 subjects were male. Subjects were randomized to receive either 60 IU/kg or 40 IU/kg HAEGARDA in one 16-week treatment period and placebo in the other 16-week treatment period. Patients self-administered HAEGARDA or placebo subcutaneously 2 times per week. Efficacy was evaluated for the last 14 weeks of each treatment period.
Eligible patients were also able to participate in Study 2 for up to 140 weeks. Approximately half of the subjects enrolled in Study 2 also participated in Study 1 (59/120, 49.2%).
Twice per week S.C. doses of 60 IU/kg or 40 IU/kg HAEGARDA resulted in a significant difference in the time-normalized number of HAE attacks (the rate of attacks) relative to placebo (Table 4). The time normalized number of HAE attacks in subjects dosed with 60 IU/kg was 0.52 attacks per month compared to 4.03 attacks per month while receiving placebo (p <0.001). The time normalized number of HAE attacks in subjects dosed with 40 IU/kg was 1.19 attacks per month compared to 3.61 attacks per month while receiving placebo (p <0.001).
Table 4. Time-normalized Number of HAE Attacks (Number/Month) 60 IU/kg HAEGARDA Treatment Sequences
(N = 45)40 IU/kg HAEGARDA Treatment Sequences
(N = 45)HAEGARDA Placebo HAEGARDA Placebo CI = confidence interval; HAE = hereditary angioedema; N = number of randomized subjects;
n = number of subjects with data; LS = Least squares.- *
- From a mixed model.
n 43 42 43 44 Mean (SD) 0.5 (0.8) 4.0 (2.3) 1.2 (2.3) 3.6 (2.1) Min, Max 0.0, 3.1 0.6, 11.3 0.0, 12.5 0.0, 8.9 Median 0.3 3.8 0.3 3.8 LS Mean (SE)* 0.5 (0.3) 4.0 (0.3) 1.2 (0.3) 3.6 (0.3) 95% CI for LS Mean* (0.0, 1.0) (3.5, 4.6) (0.5, 1.9) (3, 4.3) Treatment difference (within-subjects) 60 IU/kg HAEGARDA – Placebo 40 IU/kg HAEGARDA – Placebo LS Mean* (95% CI) -3.5 (-4.2, -2.8) -2.4 (-3.4, -1.5) p-value* < 0.001 < 0.001 The median (25th, 75th percentile) percentage reduction in the time-normalized number of HAE attacks relative to placebo was 95% (79, 100) on 60 IU/kg HAEGARDA and 89% (70, 100) on 40 IU/kg HAEGARDA among subjects with evaluable data in both treatment periods.
The percentage of responders (95% CI) with a ≥50% reduction in the time‑normalized number of HAE attacks on HAEGARDA relative to placebo was 83% (73%, 90%). Ninety percent (90%) of subjects on 60 IU/kg responded to treatment and 76% of subjects on 40 IU/kg responded to treatment.
The percentages of subjects (95% CI) with ≥70% and ≥90% reductions in the time‑normalized number of HAE attacks on HAEGARDA relative to placebo were 74% (64%, 83%) and 50% (39%, 61%), respectively. The percentages of subjects with ≥70% and ≥90% reductions in comparison to placebo in the time-normalized number of HAE attacks were 83% and 58% on 60 IU/kg and 67% and 43% on 40 IU/kg. Seventy-one percent (71%) of subjects on 60 IU/kg and 53% of subjects on 40 IU/kg had ≥1 HAE attack per 4 week period on placebo and <1 HAE attack per 4 week period on HAEGARDA.
A total of 40% of subjects on 60 IU/kg and 38% of subjects on 40 IU/kg were attack-free, and the median rate of HAE attacks per month was 0.3 on both doses.
HAEGARDA resulted in a significant difference in the time-normalized number of uses of rescue medication (the rate of rescue medication use) relative to placebo. A dose of 60 IU/kg resulted in a mean rate of rescue medication of 0.3 uses per month, compared to 3.9 uses per month with placebo. A dose of 40 IU/kg resulted in a mean rate of rescue medication use of 1.1 uses per month, compared to 5.6 uses per month with placebo.
Study 2:
The study assessed 120 adult and pediatric subjects with symptomatic HAE type I or II. The median (range) age of subjects was 41.0 (8-72) years. Patients with a monthly attack rate of 4.3 in 3 months before entry in the study were enrolled and treated for a mean of 1.4 years; 41 patients (34.2%) had more than 2 years of exposure. Mean steady-state C1-INH functional activity increased to 52.0% with 40 IU/kg and 66.6% with 60 IU/kg. Incidence of adverse events was similar in both dose groups (12.0 and 8.6 events per patient-year for 40 IU/kg and 60 IU/kg, respectively). The percentages of subjects with ≥50% reductions in the time-normalized number of HAE attacks on HAEGARDA relative to the time-normalized number of HAE attacks at baseline were 93.1% and 93.1% in 40 IU/kg and 60 IU/kg treatment arms, respectively. The percentages of subjects with time normalized HAE attack frequency of < 1 HAE attack per 4-week period were 79.7% on 40 IU/kg and 86.9% on 60 IU/kg. For 40 IU/kg and 60 IU/kg, median annualized attack rates were 1.0 and 1.0, respectively, and median rescue medication use was 0.0 and 0.0 times per year, respectively. The proportion of HAE attack-free subjects throughout the study duration with a maximum exposure of >2.5 years was 35.6% and 44.3% in the 40 IU/kg and 60 IU/kg treatment arms, respectively.
-
15 REFERENCES
- Martinez-Saguer I, Rusicke E, Aygören-Pürsün E, et al. Characterization of acute hereditary angioedema attacks during pregnancy and breast-feeding and their treatment with C1 inhibitor concentrate. Am J Obstet Gynecol. 2010;203:131.e1-7.
- Fox J, Vegh AB, Martinez-Saguer I, et al. Safety of a C1-inhibitor concentrate in pregnant women with hereditary angioedema. Allergy Asthma Proc. 2017;38(3):216-221
-
16 HOW SUPPLIED/STORAGE AND HANDLING
HAEGARDA is supplied in a kit containing a lyophilized powder in a single-dose vial.
HAEGARDA is packaged with Sterile Water for Injection, USP (4 mL for reconstitution of 2000 IU or 5.6 mL for reconstitution of 3000 IU) and one Mix2Vial filter transfer set. Not made with natural rubber latex.
Table 5. How Supplied Nominal Strength Fill Size Color Indicator Kit NDC 2000 IU Fuschia 63833-828-02 3000 IU Yellow 63833-829-02 Storage and Handling
- When stored at temperatures up to 30°C (86°F), HAEGARDA is stable for the period indicated by the expiration date on the carton and vial label.
- Keep HAEGARDA in its original carton until ready to use.
- Do not freeze.
- Protect from light.
- Discard any unused product and all used disposable supplies.
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Product Information).
All risks and benefits of HAEGARDA should be discussed with the patient/caregiver before prescribing or administering it to the patient.
Inform patients/caregivers to immediately report the following to their physician:
- Signs and symptoms of allergic hypersensitivity reactions, such as hives, tightness of the chest, difficulty breathing, wheezing, hypotension and/or anaphylaxis experienced during or after injection of HAEGARDA [see Warnings and Precautions (5.1)].
- Signs and symptoms of a thromboembolic event, including pain and/or swelling of an arm or leg with warmth over the affected area, discoloration of an arm or leg, unexplained shortness of breath, chest pain or discomfort that worsens on deep breathing, unexplained rapid pulse, numbness or weakness on one side of the body [see Warnings and Precautions (5.2)].
Inform all patients/caregivers:
- HAEGARDA is indicated for HAE prophylaxis and should not be used for the treatment of acute HAE attacks. Patients/caregivers should be counselled regarding the appropriate course of action if breakthrough HAE attacks occur while on HAEGARDA, including:
- Individualized rescue treatment for acute HAE attacks.
- Situations in which to seek immediate medical attention, such as acute laryngeal HAE attacks.
- Patients/caregivers must ensure an adequate supply of HAEGARDA when traveling.
- Because HAEGARDA is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent [see Warnings and Precautions (5.3) and Description (11)].
- Patients with known risk factors for thromboembolic events are at an increased risk for these events [see Warnings and Precautions (5.2)].
- Ensure that the patient/caregiver has access to and has received training in the administration of subcutaneous epinephrine and/or other appropriate supportive therapy for the treatment of any acute anaphylactic or severe hypersensitivity reaction [see Warnings and Precautions (5.1)].
Advise female patients:
- Patients should notify their physician if they become pregnant or intend to become pregnant while taking HAEGARDA [see Use in Specific Populations (8.1)].
- Patients should notify their physician if they are breastfeeding or plan to breastfeed while taking HAEGARDA [see Use in Specific Populations (8.2)].
Self-administration - Ensure that the patient/caregiver receives clear instructions and training on S.C. administration in the home or other appropriate setting and has demonstrated the ability to perform S.C. injection.
- Ensure the patient (or caregiver) has the necessary dexterity and comprehension to be trained to self-administer.
- Instruct patients/caregivers to record the lot number from the HAEGARDA vial label every time they use HAEGARDA.
The attached HAEGARDA "Patient Product Information (PPI)" contains more detailed instructions for patients/caregivers who will be self-administering HAEGARDA.
- SPL UNCLASSIFIED SECTION
-
FDA-Approved Patient Labeling – Patient Product Information (PPI)
HAEGARDA (hay-GAR-duh)
C1 Esterase Inhibitor Subcutaneous (Human)
Freeze-Dried Powder for ReconstitutionThis leaflet summarizes important information about HAEGARDA. Please read it carefully before using HAEGARDA and each time you get a refill. There may be new information provided. This information does not take the place of talking with your healthcare provider, and it does not include all of the important information about HAEGARDA. If you have any questions after reading this, ask your healthcare provider.
Do not attempt to self-administer unless you have been taught how by your healthcare provider.
What is HAEGARDA?
HAEGARDA is an injectable medicine used to prevent swelling and/or painful attacks in patients 6 years of age and older with Hereditary Angioedema (HAE). HAE is caused by the poor functioning or lack of a protein called C1 that is present in your blood and helps control inflammation (swelling) and parts of the immune system. HAEGARDA contains C1 esterase inhibitor (C1-INH), a protein that helps control C1.
HAEGARDA should not be used to treat an acute HAE attack. In case of an acute HAE attack, initiate individualized treatment as discussed with your prescribing health care professional.
Who should not use HAEGARDA?
You should not use HAEGARDA if you have experienced life-threatening immediate hypersensitivity reactions, including anaphylaxis, to the product.
What should I tell my healthcare provider before using HAEGARDA?
Tell your healthcare provider about all of your medical conditions, including if you:
- Are pregnant or planning to become pregnant. It is not known if HAEGARDA can harm your unborn baby.
- Are breastfeeding or plan to breastfeed. It is not known if HAEGARDA passes into your milk and if it can harm your baby.
- Have a history of blood clotting problems. Blood clots have occurred in patients receiving HAEGARDA. Very high doses of C1-INH could increase the risk of blood clots. Tell your healthcare provider if you have a history of heart or blood vessel disease, stroke, blood clots, or have thick blood, an indwelling catheter/access device in one of your veins, or have been immobile for some time. These things may increase your risk of having a blood clot after using HAEGARDA. Also, tell your healthcare provider what drugs you are using, as some drugs, such as birth control pills or certain androgens, may increase your risk of developing a blood clot.
Tell your healthcare provider and pharmacist about all of the medicines you take, including all prescription and non-prescription medicines such as over-the-counter medicines, supplements, or herbal remedies.
What are the possible side effects of HAEGARDA?
Allergic reactions may occur with HAEGARDA. Call your healthcare provider or seek emergency support services right away if you have any of the following symptoms after using HAEGARDA:
- wheezing
- difficulty breathing
- chest tightness
- turning blue (look at lips and gums)
- fast heartbeat
- swelling of the face
- rash or hives
Signs of a blood clot include:
- pain and/or swelling of an arm or leg with warmth over the affected area
- discoloration of an arm or leg
- unexplained shortness of breath
- chest pain or discomfort that worsens on deep breathing
- unexplained rapid pulse
- numbness or weakness on one side of the body
Because HAEGARDA is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent.
The most common side effects with HAEGARDA are injection site reactions (pain, redness, swelling), hypersensitivity (itching and rash), nasopharyngitis (runny or stuffy nose, sneezing, watery eyes) and dizziness.
These are not all the possible side effects of HAEGARDA.
Tell your healthcare provider about any side effect that bothers you or that does not go away. You can also report side effects to the FDA at 1-800-FDA-1088.
How should I store HAEGARDA?
- Keep the non-reconstituted HAEGARDA in its original carton to protect from light until ready to use.
- When stored at temperatures up to 30°C (86°F), HAEGARDA is stable for the period indicated by the expiration date on the carton and vial label.
- Do not freeze.
What else should I know about HAEGARDA?
Medicines are sometimes prescribed for purposes other than those listed here. Do not use HAEGARDA for a condition for which it is not prescribed. Do not share HAEGARDA with other people, even if they have the same symptoms that you have.
This leaflet summarizes the most important information about HAEGARDA. If you would like more information, talk to your healthcare provider. You can ask your healthcare provider or pharmacist for information about HAEGARDA that was written for healthcare professionals. For more information, go to www.HAEGARDA.com or call 1-877-236-4423.
What should I know about self-administration?
- You should prepare the prescribed dose of HAEGARDA for self-administration as directed by your healthcare provider.
-
Instructions for Use
- Do not attempt to self-administer unless you have been taught how by your healthcare provider.
- See the step-by-step instructions for injecting HAEGARDA at the end of this leaflet. You should always follow the specific instructions given by your healthcare provider. The steps listed below are general guidelines for using HAEGARDA. If you are unsure of the steps, please contact your healthcare provider or pharmacist before using.
- Your healthcare provider will prescribe the dose that you should administer, which is based on your body weight.
- Call your healthcare provider if you miss a dose of HAEGARDA.
- Talk to your healthcare provider before traveling to make sure you have an adequate supply of HAEGARDA.
- Use a new needle for each HAEGARDA injection. Do not reuse or share your needles with other people. You may give other people a serious infection, or get a serious infection from them.
Reconstitution and Administration
- The 2000 IU HAEGARDA vial contains C1-INH as a lyophilized concentrate for reconstitution with 4 mL of Sterile Water for Injection, USP provided; or, the 3000 IU HAEGARDA vial contains C1-INH as a lyophilized concentrate for reconstitution with 5.6 mL of Sterile Water for Injection, USP provided.
- Check the expiration date on the product vial label. Do not use beyond the expiration date.
- Work on a clean surface and wash hands before performing the following procedures.
- Use either the Mix2Vial transfer set provided with HAEGARDA or a commercially available double-ended needle and vented filter spike.
- Prepare and administer using aseptic techniques.
- Each vial of HAEGARDA is for single-dose only. Promptly use the reconstituted solution. The solution must be used within 8 hours. Discard partially used vials. HAEGARDA contains no preservative.
- After reconstitution and prior to administration inspect HAEGARDA. The reconstituted solution should be colorless, clear, and free from visible particles. Do not use if the solution is cloudy, discolored, or contains particulates.
Reconstitution
The procedures below are provided as general guidelines for the reconstitution of HAEGARDA.
HAEGARDA Reconstitution Instructions
- 1.
- Ensure that the HAEGARDA vial and Sterile Water for Injection (diluent) vial are at room temperature.
- 2.
- Place the HAEGARDA vial, diluent vial and Mix2Vial transfer set on a flat surface.
- 3.
- Remove flip caps from the HAEGARDA and diluent vials.
- 4.
- Wipe the stoppers with an alcohol swab and allow to dry prior to opening the Mix2Vial transfer set package.
- 5.
- Open the Mix2Vial transfer set package by peeling away the lid (Figure 1). Do not remove the device from the package.
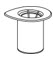
Figure 1 - 6.
- Place the diluent vial on a flat surface and hold the vial tightly. Grip the Mix2Vial transfer set together with the clear package and push the plastic spike at the blue end of the Mix2Vial transfer set firmly through the center of the stopper of the diluent vial (Figure 2).
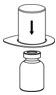
Figure 2 - 7.
- Carefully remove the clear package from the Mix2Vial transfer set. Do not remove the Mix2Vial transfer set or touch the exposed end of the device (Figure 3).
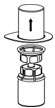
Figure 3 - 8.
- With the HAEGARDA vial placed firmly on a flat surface, invert the diluent vial with the Mix2Vial transfer set attached and push the plastic spike of the transparent adapter firmly through the center of the stopper of the HAEGARDA vial (Figure 4). The diluent will automatically transfer into the HAEGARDA vial.
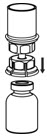
Figure 4 - 9.
- With the diluent and HAEGARDA vial still attached to the Mix2Vial transfer set, gently swirl the HAEGARDA vial to ensure that the powder is fully dissolved (Figure 5). Do not shake the vial.
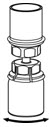
Figure 5 - 10.
- With one hand, grasp the HAEGARDA side of the Mix2Vial transfer set and with the other hand grasp the blue diluent side of the Mix2Vial transfer set, and unscrew the set into two pieces (Figure 6).
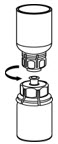
Figure 6 - 11.
- Draw air into an empty, sterile syringe. Use a silicone-free syringe. While the HAEGARDA vial is upright, screw the syringe to the Mix2Vial transfer set. Inject air into the HAEGARDA vial.
- 12.
- While keeping the syringe plunger pressed, invert the system upside down and draw the concentrate into the syringe by pulling the plunger back slowly (Figure 7).
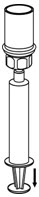
Figure 7 - 13.
- Disconnect the filled syringe by unscrewing it from the Mix2Vial transfer set (Figure 8). The reconstituted solution should be colorless, clear, and free from visible particles. Do not use if particles or discoloration are observed.
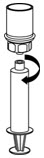
Figure 8 - 14.
- Use immediately or within 8 hours of reconstitution. Store reconstituted solution at room temperature. Do not refrigerate.
- 15.
- If the dose requires more than one vial, use a separate, unused Mix2Vial transfer set and diluent vial for each product vial. Repeat steps 10-12 to pool the contents of the vials into one syringe.
Self (or caregiver)-Administration (subcutaneous administration)
Your healthcare provider will teach you (or caregiver) how to safely administer HAEGARDA. Once you (or caregiver) learn how to administer, follow the instructions provided below.
HAEGARDA administration instructions apply to patients, 6 years of age and older.
HAEGARDA Self (or caregiver)-Administration Instructions
Step 1: Assemble supplies
Gather the HAEGARDA syringe, the following disposable supplies (not provided with HAEGARDA), and other items (sharps or other container, treatment diary or log book):
- Hypodermic needle or S.C. infusion set
- Sterile syringe (Use a silicone-free syringe)
- Alcohol wipes
- Gloves (if recommended by the healthcare provider)
Step 2: Clean surface
- Thoroughly clean a table or other flat surface using alcohol wipes.
Step 3: Wash hands
- Thoroughly wash and dry your hands.
- If you have been told to wear gloves when preparing the infusion, put the gloves on.
Step 4: Prepare injection site
- Select an area on the abdomen (stomach) or another site for the injection as discussed with your doctor (Figure 9).
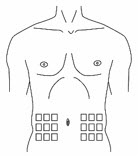
Figure 9 - Use a different place from the last injection; you should rotate the places where you are injecting.
- New injection sites should be at least 2 inches (5 centimeters) away from the place where you gave yourself an injection before.
- Never give an injection in areas where the skin is itchy, swollen, painful, bruised, or red.
- Avoid giving injections in places where you have scars or stretch marks.
- Clean the skin at the injection site with an alcohol swab and let the skin dry (Figure 10).
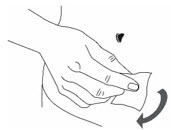
Figure 10 Step 5: Injection in the abdominal area
As instructed by the healthcare provider:
- Attach a hypodermic needle or S.C. infusion set (butterfly) as instructed by the healthcare provider. Prime the needle or tubing as required and instructed.
Injection with Hypodermic Needle:
- Insert the needle into the fold of skin (Figure 11).
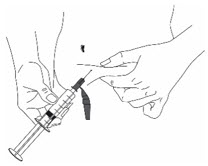
Figure 11 Injection by S.C Infusion Set:
- Insert the needle into the fold of skin (Figure 12).
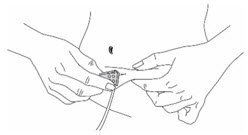
Figure 12 Step 6: Clean up
- After injecting the entire amount of HAEGARDA, remove the needle.
- Discard any unused solution and all administration equipment in an appropriate manner as per local requirements.
Step 7: Record treatment
Record the lot number from the HAEGARDA vial label in the treatment diary or log book with the date and time of infusion every time you use HAEGARDA.
Resources at CSL Behring available to the patient:
For Adverse Reaction Reporting contact:
CSL Behring Pharmacovigilance Department at 1-866-915-6958Contact CSL Behring to receive more product information:
Customer Support 1-800-683-1288For more information, visit www.HAEGARDA.com.
Manufactured by:
CSL Behring GmbH
35041 Marburg, Germany
US License No. 1765Distributed by:
CSL Behring LLC
Kankakee, IL 60901 USAMix2Vial® is a registered trademark of West Pharma. Services IL, Ltd., a subsidiary of West Pharmaceuticals Services, Inc.
- PRINCIPAL DISPLAY PANEL - Kit Carton - 2000 IU
- PRINCIPAL DISPLAY PANEL - Kit Carton - 3000 IU
-
INGREDIENTS AND APPEARANCE
HAEGARDA C1 ESTERASE INHIBITOR SUBCUTANEOUS (HUMAN)
human c1-esterase inhibitor kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:63833-828 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63833-828-02 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 4 mL Part 2 1 VIAL, SINGLE-USE 4 mL Part 1 of 2 HAEGARDA C1 ESTERASE INHIBITOR SUBCUTANEOUS (HUMAN)
human c1-esterase inhibitor injection, powder, for solutionProduct Information Item Code (Source) NDC:63833-838 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN C1-ESTERASE INHIBITOR (UNII: 6KIC4BB60G) (HUMAN C1-ESTERASE INHIBITOR - UNII:6KIC4BB60G) HUMAN C1-ESTERASE INHIBITOR 2000 [iU] in 4 mL Inactive Ingredients Ingredient Name Strength Glycine (UNII: TE7660XO1C) 40 mg in 4 mL Sodium Chloride (UNII: 451W47IQ8X) 34 mg in 4 mL Sodium Citrate, Unspecified Form (UNII: 1Q73Q2JULR) 10 mg in 4 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63833-838-01 4 mL in 1 VIAL, SINGLE-USE; Type 6: Drug/Biologic Combination Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125606 06/22/2017 Part 2 of 2 STERILE WATER
water injection, solutionProduct Information Item Code (Source) NDC:63833-765 Route of Administration SUBCUTANEOUS Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63833-765-18 4 mL in 1 VIAL, SINGLE-USE; Type 6: Drug/Biologic Combination Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125606 06/22/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125606 06/22/2017 HAEGARDA C1 ESTERASE INHIBITOR SUBCUTANEOUS (HUMAN)
human c1-esterase inhibitor kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:63833-829 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63833-829-02 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 6 mL Part 2 1 VIAL, SINGLE-USE 6 mL Part 1 of 2 HAEGARDA C1 ESTERASE INHIBITOR SUBCUTANEOUS (HUMAN)
human c1-esterase inhibitor injection, powder, for solutionProduct Information Item Code (Source) NDC:63833-839 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN C1-ESTERASE INHIBITOR (UNII: 6KIC4BB60G) (HUMAN C1-ESTERASE INHIBITOR - UNII:6KIC4BB60G) HUMAN C1-ESTERASE INHIBITOR 3000 [iU] in 6 mL Inactive Ingredients Ingredient Name Strength Glycine (UNII: TE7660XO1C) 60 mg in 6 mL Sodium Chloride (UNII: 451W47IQ8X) 51 mg in 6 mL Sodium Citrate, Unspecified Form (UNII: 1Q73Q2JULR) 15 mg in 6 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63833-839-01 5.6 mL in 1 VIAL, SINGLE-USE; Type 6: Drug/Biologic Combination Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125606 06/22/2017 Part 2 of 2 STERILE WATER
water injection, solutionProduct Information Item Code (Source) NDC:63833-765 Route of Administration SUBCUTANEOUS Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63833-765-19 5.6 mL in 1 VIAL, SINGLE-USE; Type 6: Drug/Biologic Combination Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125606 06/22/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125606 06/22/2017 Labeler - CSL Behring GmbH (326530474) Establishment Name Address ID/FEI Business Operations CSL Behring GmbH 326530474 MANUFACTURE




