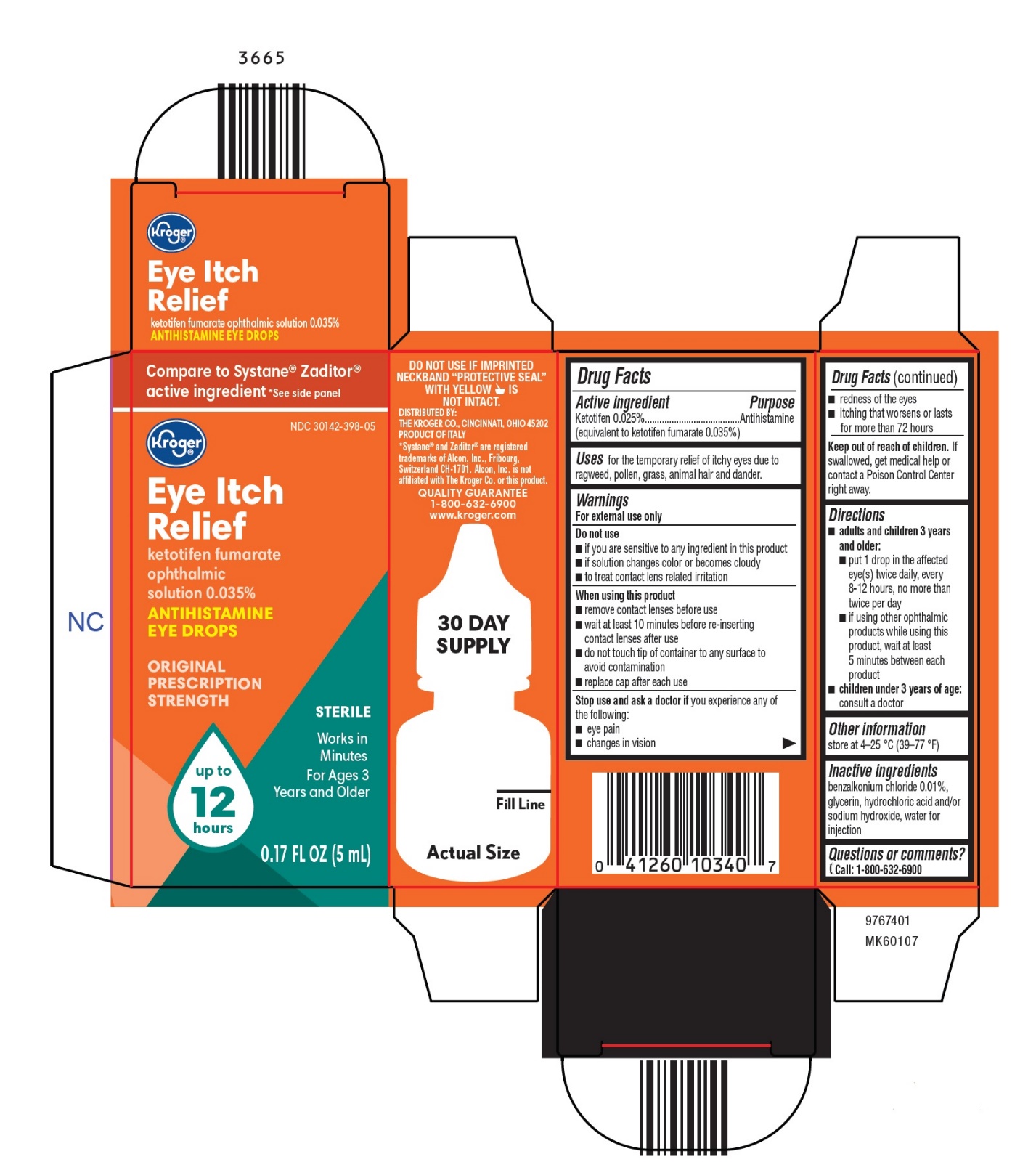
Label: EYE ITCH RELIEF- ketotifen fumarate solution/ drops
- NDC Code(s): 30142-398-05
- Packager: Kroger Company
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 8, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- •
- if you are sensitive to any ingredient in this product
- •
- if solution changes color or becomes cloudy
- •
- to treat contact lens related irritation
When using this product
- •
- remove contact lenses before use
- •
- wait at least 10 minutes before re-inserting contact lenses after use
- •
- do not touch tip of container to any surface to avoid contamination
- •
- replace cap after each use
- Directions
-
Inactive ingredients
benzalkonium chloride 0.01%, glycerin, hydrochloric acid and/or sodium hydroxide, water for injection
Questions or comments?
[phone icon]Call: 1-800-632-6900
DISTRIBUTED BY:
THE KROGER CO., CINCINNATI, OHIO 45202
PRODUCT OF ITALY*Systane® and Zaditor® are registered
trademarks of Alcon, Inc., Fribourg,
Switzerland CH-1701. Alcon, Inc. is not
affiliated with The Kroger Co. or this product.QUALITY GUARANTEE
1-800-632-6900
www.kroger.com9767401
MK60107
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
EYE ITCH RELIEF
ketotifen fumarate solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:30142-398 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength KETOTIFEN FUMARATE (UNII: HBD503WORO) (KETOTIFEN - UNII:X49220T18G) KETOTIFEN 0.25 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) GLYCERIN (UNII: PDC6A3C0OX) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:30142-398-05 1 in 1 CARTON 06/01/2022 1 5 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021996 06/01/2022 Labeler - Kroger Company (006999528) Establishment Name Address ID/FEI Business Operations Bausch & Lomb Incorporated 079587625 MANUFACTURE(30142-398)