Label: ALBENDAZOLE tablet, film coated
- NDC Code(s): 0115-1701-49
- Packager: Amneal Pharmaceuticals of New York LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 30, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ALBENDAZOLE TABLETS safely and effectively. See full prescribing information for ALBENDAZOLE TABLETS.
ALBENDAZOLE tablets, for oral use
Initial U.S. Approval: 1996INDICATIONS AND USAGE
Albendazole is an anthelmintic drug indicated for:
DOSAGE AND ADMINISTRATION
Patients weighing 60 kg or greater, 400 mg twice daily; less than 60 kg, 15 mg/kg/day in divided doses twice daily (maximum total daily dose 800 mg). Albendazole tablets should be taken with food. (2)
- Hydatid disease: 28-day cycle followed by 14-day albendazole-free interval for a total of 3 cycles. (2)
- Neurocysticercosis: 8 to 30 days. (2)
See additional important information in the Full Prescribing Information. (2)
DOSAGE FORMS AND STRENGTHS
- Tablet: 200 mg (3)
CONTRAINDICATIONS
Patients with known hypersensitivity to the benzimidazole class of compounds or any components of albendazole. (4)
WARNINGS AND PRECAUTIONS
- Bone Marrow Suppression: Fatalities have been reported due to bone marrow suppression; monitor blood counts in all patients at the beginning of each 28-day cycle of therapy, and every 2 weeks while on therapy. Discontinue albendazole if clinically significant changes in blood counts occur. (5.1, 5.4)
- Embryo-fetal Toxicity: Obtain pregnancy test in women of reproductive potential prior to therapy and avoid usage in pregnant women except in clinical circumstances where no alternative management is appropriate. Discontinue therapy if pregnancy occurs and apprise patient of potential hazard to the fetus. (5.2)
- Risk of Neurologic Symptoms: Neurocysticercosis patients may experience cerebral hypertensive episodes, seizures or focal neurologic deficits after initiation of therapy; begin appropriate steroid and anticonvulsant therapy. (5.3)
- Risk of Retinal Damage in Retinal Cysticercosis: Cases of retinal involvement have been reported; examine the patient for the presence of retinal lesions before initiating therapy for neurocysticercosis. (5.4)
- Hepatic Effects. Elevations of liver enzymes may occur. Monitor liver enzymes before the start of each treatment cycle and at least every 2 weeks while on albendazole therapy and discontinue if clinically significant elevations occur. (5.5)
ADVERSE REACTIONS
- Adverse reactions 1% or greater in hydatid disease: abnormal liver function tests, abdominal pain, nausea/vomiting, reversible alopecia, headache, dizziness/vertigo, fever. (6.1)
- Adverse reactions 1% or greater in neurocysticercosis: headache, nausea/vomiting, raised intracranial pressure, meningeal signs. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Dexamethasone: Steady-state trough concentrations of albendazole sulfoxide were about 56% higher when dexamethasone was coadministered with each dose of albendazole. (7.1)
- Praziquantel: In the fed state increased mean maximum plasma concentration and area under the curve of albendazole sulfoxide by about 50% in healthy subjects. (7.2)
- Cimetidine: Increased albendazole sulfoxide concentrations in bile and cystic fluid by about 2-fold in hydatid cyst patients. (7.3)
- Theophylline: Albendazole induces cytochrome P450 1A in human hepatoma cells; therefore, it is recommended that plasma concentrations of theophylline be monitored during and after treatment. (5.5, 7.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Neurocysticercosis
1.2 Hydatid Disease
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Concomitant Medication to Avoid Adverse Reactions
2.3 Monitoring for Safety Before and During Treatment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Bone Marrow Suppression
5.2 Embryo-fetal Toxicity
5.3 Risk of Neurologic Symptoms in Neurocysticercosis
5.4 Risk of Retinal Damage in Patients with Retinal Neurocysticercosis
5.5 Hepatic Effects
5.6 Unmasking of Neurocysticercosis in Hydatid Patients
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Dexamethasone
7.2 Praziquantel
7.3 Cimetidine
7.4 Theophylline
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Impaired Renal Function
8.7 Patients with Extra-Hepatic Obstruction
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
Dosing of albendazole will vary depending upon the indication. Albendazole tablets may be crushed or chewed and swallowed with a drink of water. Albendazole tablets should be taken with food [see Clinical Pharmacology (12.3)].
Table 1: Albendazole Dosage
Indication Patient Weight Dose Duration Hydatid Disease
60 kg or greater
400 mg twice daily, with meals
28-day cycle followed by a 14-day albendazole-free interval, for a total of 3 cycles
Less than 60 kg
15 mg/kg/day given in divided doses twice daily with meals (maximum total daily dose 800 mg)
Neurocysticercosis
60 kg or greater
400 mg twice daily, with meals
8 to 30 days
Less than 60 kg
15 mg/kg/day given in divided doses twice daily with meals (maximum total daily dose 800 mg)
2.2 Concomitant Medication to Avoid Adverse Reactions
Patients being treated for neurocysticercosis should receive appropriate steroid and anticonvulsant therapy as required. Oral or intravenous corticosteroids should be considered to prevent cerebral hypertensive episodes during the first week of treatment [see Warnings and Precautions (5.3)].
2.3 Monitoring for Safety Before and During Treatment
- Monitor blood counts at the beginning of each 28-day cycle of therapy, and every 2 weeks while on therapy with albendazole in all patients [see Warnings and Precautions (5.1)].
- Monitor liver enzymes (transaminases) at the beginning of each 28-day cycle of therapy, and at least every 2 weeks during treatment with albendazole in all patients [see Warnings and Precautions (5.5)].
- Obtain a pregnancy test in women of reproductive potential prior to therapy [see Warnings and Precautions (5.2)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Bone Marrow Suppression
Fatalities associated with the use of albendazole have been reported due to granulocytopenia or pancytopenia. Albendazole may cause bone marrow suppression, aplastic anemia, and agranulocytosis. Monitor blood counts at the beginning of each 28-day cycle of therapy, and every 2 weeks while on therapy with albendazole in all patients. Patients with liver disease and patients with hepatic echinococcosis are at increased risk for bone marrow suppression and warrant more frequent monitoring of blood counts. Discontinue albendazole if clinically significant decreases in blood cell counts occur.
5.2 Embryo-fetal Toxicity
Albendazole may cause fetal harm and should not be used in pregnant women except in clinical circumstances where no alternative management is appropriate. Obtain pregnancy test prior to prescribing albendazole to women of reproductive potential. Advise women of reproductive potential to use effective birth control for the duration of albendazole therapy and for one month after end of therapy. Immediately discontinue albendazole if a patient becomes pregnant and apprise the patient of the potential hazard to the fetus [see Use in Specific Populations (8.1, 8.3)].
5.3 Risk of Neurologic Symptoms in Neurocysticercosis
Patients being treated for neurocysticercosis should receive steroid and anticonvulsant therapy to prevent neurological symptoms (e.g. seizures, increased intracranial pressure and focal signs) as a result of an inflammatory reaction caused by death of the parasite within the brain.
5.4 Risk of Retinal Damage in Patients with Retinal Neurocysticercosis
Cysticercosis may involve the retina. Before initiating therapy for neurocysticercosis, examine the patient for the presence of retinal lesions. If such lesions are visualized, weigh the need for anticysticeral therapy against the possibility of retinal damage resulting from inflammatory damage caused by albendazole-induced death of the parasite.
5.5 Hepatic Effects
In clinical trials, treatment with albendazole has been associated with mild to moderate elevations of hepatic enzymes in approximately 16% of patients. These elevations have generally returned to normal upon discontinuation of therapy. There have also been case reports of acute liver failure of uncertain causality and hepatitis [see Adverse Reactions (6)].
Monitor liver enzymes (transaminases) before the start of each treatment cycle and at least every 2 weeks during treatment. If hepatic enzymes exceed twice the upper limit of normal, consideration should be given to discontinuing albendazole therapy based on individual patient circumstances. Restarting albendazole treatment in patients whose hepatic enzymes have normalized off treatment is an individual decision that should take into account the risk/benefit of further albendazole usage. Perform laboratory tests frequently if albendazole treatment is restarted.
Patients with elevated liver enzyme test results are at increased risk for hepatotoxicity and bone marrow suppression [see Warnings and Precautions (5.1)]. Discontinue therapy if liver enzymes are significantly increased or if clinically significant decreases in blood cell counts occur.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The adverse reaction profile of albendazole differs between hydatid disease and neurocysticercosis. Adverse reactions occurring with a frequency of 1% or greater in either disease are described in Table 2 below.
These symptoms were usually mild and resolved without treatment. Treatment discontinuations were predominantly due to leukopenia (0.7%) or hepatic abnormalities (3.8% in hydatid disease). The following incidence reflects adverse reactions that were reported to be at least possibly or probably related to albendazole.
Table 2: Adverse Reaction Incidence 1% or Greater in Hydatid Disease and Neurocysticercosis
Adverse Reaction
Hydatid Disease
Neurocysticercosis
Gastrointestinal
Abdominal Pain
6
0
Nausea
4
6
Vomiting
4
6
General disorders and administration site conditions
Fever
1
0
Investigations
Elevated Hepatic Enzymes
16
less than 1
Nervous system disorders
Dizziness
1
less than 1
Headache
1
11
Meningeal Signs
0
1
Raised Intracranial Pressure
0
2
Vertigo
1
less than 1
Skin and subcutaneous tissue disorders
Reversible Alopecia
2
less than 1
The following adverse events were observed at an incidence of less than 1%:
Blood and Lymphatic System Disorders: There have been reports of leukopenia, granulocytopenia, pancytopenia, agranulocytosis, or thrombocytopenia [see Warnings and Precautions (5.1)].
Immune System Disorders: Hypersensitivity reactions, including rash and urticaria.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of albendazole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: Aplastic anemia, bone marrow suppression, neutropenia.
Eye Disorders: Vision blurred.
Gastrointestinal Disorders: Diarrhea.
General System Disorders: Asthenia.
Hepatobiliary Disorders: Elevations of hepatic enzymes, hepatitis, acute liver failure.
Musculoskeletal and Connective Tissue Disorders: Rhabdomyolysis.
Nervous System Disorders: Somnolence, convulsion.
Renal and Urinary Disorders: Acute renal failure.
Skin and Subcutaneous Tissue Disorders: Erythema multiforme, Stevens-Johnson syndrome.
-
7 DRUG INTERACTIONS
7.1 Dexamethasone
Steady-state trough concentrations of albendazole sulfoxide were about 56% higher when 8 mg dexamethasone was co-administered with each dose of albendazole (15 mg/kg/day) in 8 neurocysticercosis patients.
7.2 Praziquantel
In the fed state, praziquantel (40 mg/kg) increased mean maximum plasma concentration and area under the curve of albendazole sulfoxide by about 50% in healthy subjects (n = 10) compared with a separate group of subjects (n = 6) given albendazole alone. Mean Tmax and mean plasma elimination half-life of albendazole sulfoxide were unchanged. The pharmacokinetics of praziquantel were unchanged following co-administration with albendazole (400 mg).
7.3 Cimetidine
Albendazole sulfoxide concentrations in bile and cystic fluid were increased (about 2-fold) in hydatid cyst patients treated with cimetidine (10 mg/kg/day) (n = 7) compared with albendazole (20 mg/kg/day) alone (n = 12). Albendazole sulfoxide plasma concentrations were unchanged 4 hours after dosing.
7.4 Theophylline
Following a single dose of albendazole (400 mg), the pharmacokinetics of theophylline (aminophylline 5.8 mg/kg infused over 20 minutes) were unchanged. Albendazole induces cytochrome P450 1A in human hepatoma cells; therefore, it is recommended that plasma concentrations of theophylline be monitored during and after treatment.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are limited data on use of albendazole in pregnant women to inform a drug-associated risk of major birth defects and miscarriage. In published studies, single-dose albendazole exposure during pregnancy did not show evidence of an increased risk of adverse maternal or fetal outcomes; however, this finding cannot be extrapolated to multiple-dose exposures (see Data).
In animal reproductive studies, oral administration of albendazole during gestation caused embryotoxicity and skeletal malformations in pregnant rats (at oral doses of 0.10 times and 0.32 times the recommended human dose based on body surface area in mg/m2) and pregnant rabbits (at oral doses of 0.60 times the recommended human dose based on body surface area in mg/m2). Albendazole was also associated with maternal toxicity in rabbits (at doses of 0.60 times the recommended human dose based on body surface area in mg/m2) (see Data).
Albendazole should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Albendazole should not be used in pregnant women except in clinical circumstances where no alternative management is appropriate. If a patient becomes pregnant while taking this drug, albendazole should be discontinued immediately. If pregnancy occurs while taking this drug, the patient should be apprised of the potential hazard to the fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
A Cochrane review could not provide sufficient evidence of the impact of antihelminthics (including albendazole) on the pregnancy outcomes of low birthweight, perinatal mortality and preterm birth. In a large trial of about 2507 women, albendazole use during the second or third trimester of pregnancy had no overall effect on birth weight, perinatal mortality, or congenital anomalies. With a limited sample size and single-does exposure, another study could not rule out a two-fold increased risk of major malformations [4.7% vs. 2.2%; OR 2.2 (95% confidence interval (CI) 0.5 to 10.1); p = 0.26].
Animal Data
Albendazole has been shown to be teratogenic (to cause embryotoxicity and skeletal malformations) in pregnant rats and rabbits. The teratogenic response in the rat was shown at oral doses of 10 and 30 mg/kg/day (0.10 times and 0.32 times the recommended human dose based on body surface area in mg/m2, respectively) during gestation days 6 to 15 and in pregnant rabbits at oral doses of 30 mg/kg/day (0.60 times the recommended human dose based on body surface area in mg/m2) administered during gestation days 7 to 19. In the rabbit study, maternal toxicity (33% mortality) was noted at 30 mg/kg/day. In mice, no teratogenic effects were observed at oral doses up to 30 mg/kg/day (0.16 times the recommended human dose based on body surface area in mg/m2), administered during gestation days 6 to 15.
8.2 Lactation
Risk Summary
There are no data on the presence of albendazole in human milk, the effects on the breast-fed infant or the effects on milk production. Albendazole is excreted in animal milk.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for albendazole and any potential adverse effects on the breastfed child from albendazole or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Obtain pregnancy test prior to prescribing albendazole to women of reproductive potential.
Contraception
Females
Advise women of reproductive potential to use effective birth control for the duration of albendazole therapy and for one month after end of therapy.
8.4 Pediatric Use
Hydatid disease is uncommon in infants and young children. In neurocysticercosis, the efficacy of albendazole in children appears to be similar to that in adults.
8.5 Geriatric Use
In patients aged 65 and older with either hydatid disease or neurocysticercosis, there was insufficient data to determine whether the safety and effectiveness of albendazole is different from that of younger patients.
8.6 Patients with Impaired Renal Function
The pharmacokinetics of albendazole in patients with impaired renal function has not been studied.
8.7 Patients with Extra-Hepatic Obstruction
In patients with evidence of extrahepatic obstruction (n = 5), the systemic availability of albendazole sulfoxide was increased, as indicated by a 2-fold increase in maximum serum concentration and a 7-fold increase in area under the curve. The rate of absorption/conversion and elimination of albendazole sulfoxide appeared to be prolonged with mean Tmax and serum elimination half-life values of 10 hours and 31.7 hours, respectively. Plasma concentrations of parent albendazole were measurable in only 1 of 5 patients.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Albendazole is an orally administered anthelmintic drug. Chemically, it is methyl 5-(propylthio)-2-benzimidazolecarbamate. Its molecular formula is C12H15N3O2S. Its molecular weight is 265.34. It has the following chemical structure:
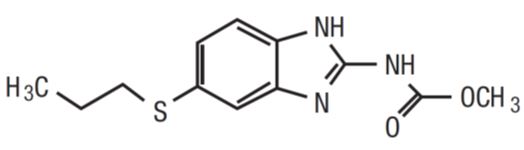
Albendazole is a white to yellowish powder. It is freely soluble in anhydrous formic acid and very slightly soluble in ether and in methylene chloride. Albendazole is practically insoluble in alcohol and in water.
Each white to off-white, circular, biconvex, bevel-edged film coated, TILTAB tablet is debossed with “ap” and “550” and contains 200 mg of albendazole.
Inactive ingredients consist of: carnauba wax, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, sodium lauryl sulfate, sodium saccharin, sodium starch glycolate, and starch.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Albendazole is a synthetic, antihelminthic drug of the class benzimidazole [see Clinical Pharmacology (12.4)].
12.3 Pharmacokinetics
Absorption
Albendazole is poorly absorbed from the gastrointestinal tract due to its low aqueous solubility. Albendazole concentrations are negligible or undetectable in plasma as it is rapidly converted to the sulfoxide metabolite prior to reaching the systemic circulation. The systemic anthelmintic activity has been attributed to the primary metabolite, albendazole sulfoxide. Oral bioavailability appears to be enhanced when albendazole is coadministered with a fatty meal (estimated fat content 40 grams) as evidenced by higher (up to 5-fold on average) plasma concentrations of albendazole sulfoxide as compared to the fasted state.
Maximal plasma concentrations of albendazole sulfoxide were achieved 2 hours to 5 hours after dosing and were on average 1310 ng/mL (range 460 ng/mL to 1580 ng/mL) following oral doses of albendazole (400 mg) in 6 hydatid disease patients, when administered with a fatty meal. Plasma concentrations of albendazole sulfoxide increased in a dose-proportional manner over the therapeutic dose range following ingestion of a high-fat meal (fat content 43.1 grams). The mean apparent terminal elimination half-life of albendazole sulfoxide ranged from 8 hours to 12 hours in 25 healthy subjects, as well as in 14 hydatid and 8 neurocysticercosis patients.
Following 4 weeks of treatment with albendazole (200 mg three times daily), 12 patients’ plasma concentrations of albendazole sulfoxide were approximately 20% lower than those observed during the first half of the treatment period, suggesting that albendazole may induce its own metabolism.
Distribution
Albendazole sulfoxide is 70% bound to plasma protein and is widely distributed throughout the body; it has been detected in urine, bile, liver, cyst wall, cyst fluid, and cerebrospinal fluid (CSF). Concentrations in plasma were 3-fold to 10-fold and 2-fold to 4-fold higher than those simultaneously determined in cyst fluid and CSF, respectively.
Metabolism and Excretion
Albendazole is rapidly converted in the liver to the primary metabolite, albendazole sulfoxide, which is further metabolized to albendazole sulfone and other primary oxidative metabolites that have been identified in human urine. Following oral administration, albendazole has not been detected in human urine. Urinary excretion of albendazole sulfoxide is a minor elimination pathway with less than 1% of the dose recovered in the urine. Biliary elimination presumably accounts for a portion of the elimination as evidenced by biliary concentrations of albendazole sulfoxide similar to those achieved in plasma.
Specific Populations
Pediatrics
Following single-dose administration of 200 mg to 300 mg (approximately 10 mg/kg) albendazole to 3 fasted and 2 fed pediatric patients with hydatid cyst disease (age range 6 to 13 years), albendazole sulfoxide pharmacokinetics were similar to those observed in fed adults.
Geriatrics
Although no studies have investigated the effect of age on albendazole sulfoxide pharmacokinetics, data in 26 hydatid cyst patients (up to 79 years) suggest pharmacokinetics similar to those in young healthy subjects.
12.4 Microbiology
Mechanism of Action
Albendazole binds to the colchicine-sensitive site of β-tubulin inhibiting their polymerization into microtubules. The decrease in microtubules in the intestinal cells of the parasites decreases their absorptive function, especially the uptake of glucose by the adult and larval forms of the parasites, and also depletes glycogen storage. Insufficient glucose results in insufficient energy for the production of adenosine trisphosphate (ATP) and the parasite eventually dies.
Mechanism of Resistance
Parasitic resistance to albendazole is caused by changes in amino acids that result in changes in the β-tubulin protein. This causes reduced binding of the drug to β-tubulin.
In the specified treatment indications albendazole appears to be active against the larval forms of the following organisms:
Echinococcus granulosus
Taenia solium
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies were conducted in mice and rats.
No evidence of increased incidence of tumors was found in the mice or rats at up to 400 mg/kg/day or 20 mg/kg/day respectively (2 times and 0.2 times the recommended human dose on a body surface area basis).
In genotoxicity tests, albendazole was found negative in an Ames Salmonella/Microsome Plate mutation assay, Chinese Hamster Ovary chromosomal aberration test, and in vivo mouse micronucleus test. In the in vitro BALB/3T3 cells transformation assay, albendazole produced weak activity in the presence of metabolic activation while no activity was found in the absence of metabolic activation.
Albendazole did not adversely affect male or female fertility in the rat at an oral dose of 30 mg/kg/day (0.32 times the recommended human dose based on body surface area in mg/m2).
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Patients should be advised that:
- Some people, particularly children, may experience difficulties swallowing the albendazole tablets whole.
- Take albendazole with food.
- Albendazole may cause fetal harm, therefore, obtain a pregnancy test in women of reproductive potential prior to initiating therapy [see Use in Specific Populations (8.1, 8.3)].
- Advise women of reproductive potential to use effective birth control while on albendazole and for one month after completing treatment [see Use in Specific Populations (8.3)].
- During albendazole therapy, monitor blood counts and liver enzymes every 2 weeks because of the possibility of harm to the liver or bone marrow [see Warnings and Precautions (5.5)].
TILTAB is a registered trademark of GlaxoSmithKline, used with permission.
Manufactured by:
GlaxoSmithKline
Mississauga, Ontario
L5N 6L4 CanadaDistributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 088071905-03
Rev. 09-2019-01
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALBENDAZOLE
albendazole tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0115-1701 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALBENDAZOLE (UNII: F4216019LN) (ALBENDAZOLE - UNII:F4216019LN) ALBENDAZOLE 200 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SACCHARIN SODIUM MONOHYDRATE (UNII: A9CO00M9HV) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white (white to off-white) Score no score Shape ROUND (cirular) Size 12mm Flavor Imprint Code ap;550 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0115-1701-49 2 in 1 BOTTLE; Type 0: Not a Combination Product 06/11/1996 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020666 06/11/1996 Labeler - Amneal Pharmaceuticals of New York LLC (123797875) Establishment Name Address ID/FEI Business Operations GlaxoSmithKline Inc. 205556368 analysis(0115-1701) , manufacture(0115-1701) , pack(0115-1701)


