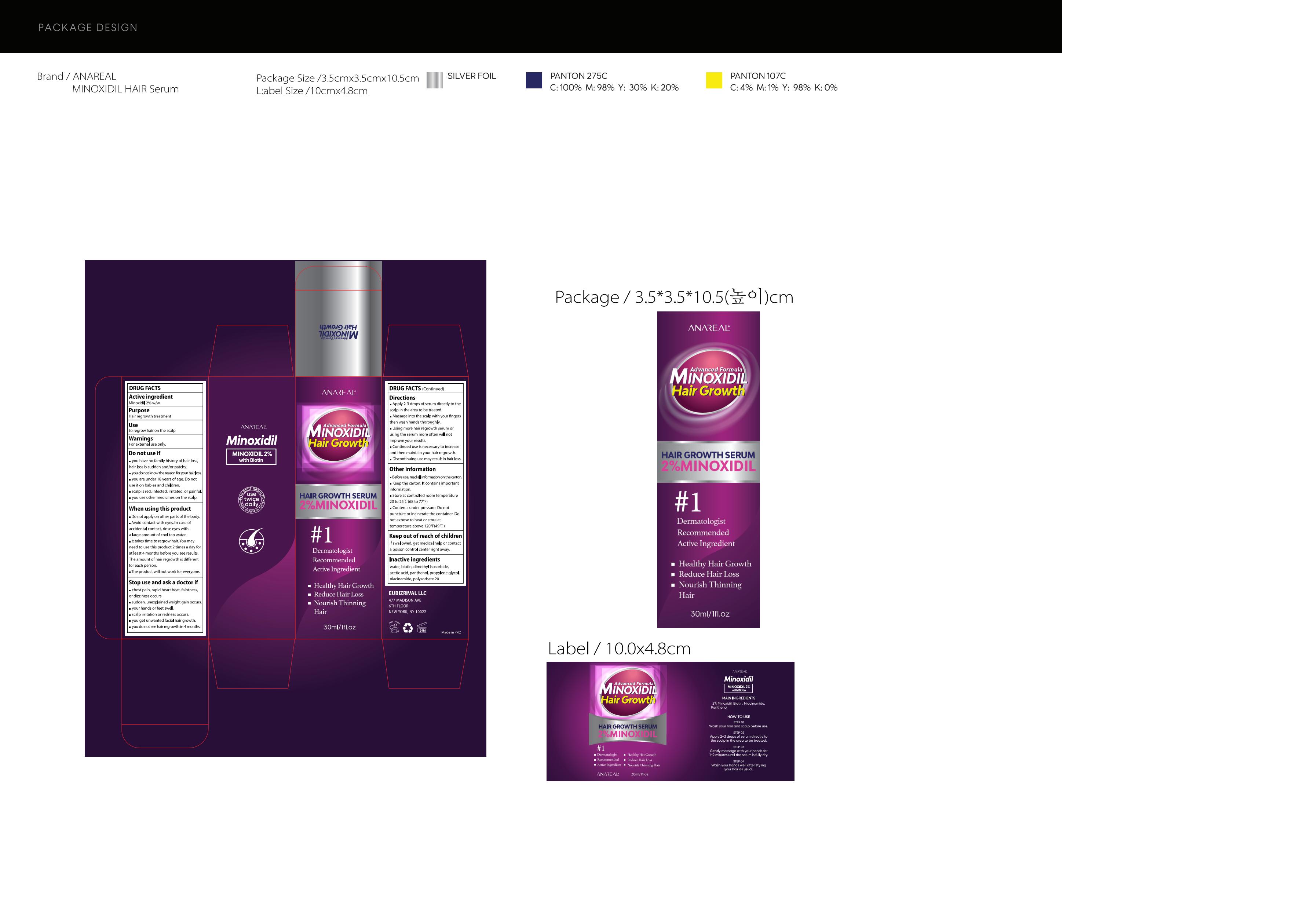
Label: 2% MINOXIDIL HAIR GROWTH OIL liquid
- NDC Code(s): 83462-007-01
- Packager: Eubizrival LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
-
When Using
Do not apply on other parts of the body. Avoid contact with eyes. In case of accidental contact, rinse eyes with a large amount of cool tap water. It takes time to regrow hair. You may need to use this product 2 times a day for at least 4 months before you see results, The amount of hair regrowth is different for each person.The product will not work for everyone.
- Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
-
Directions
. Apply 2-3 drops of serum directly to the
scalp in the area to be treated.
. Massage into the scalp with your fingers
then wash hands thoroughly.
- Using more hair regrowth serum or
using the serum more often will not
improve your results.
. Continued use is necessary to increase
and then maintain your hair regrowth.
. Discontinuing use may result in hair loss. - Other information
- Inactive ingredients
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
2% MINOXIDIL HAIR GROWTH OIL
2% minoxidil hair growth oil liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83462-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINOXIDIL (UNII: 5965120SH1) (MINOXIDIL - UNII:5965120SH1) MINOXIDIL 2 g in 100 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYSORBATE 20 (UNII: 7T1F30V5YH) NIACINAMIDE (UNII: 25X51I8RD4) WATER (UNII: 059QF0KO0R) ACETIC ACID (UNII: Q40Q9N063P) PANTHENOL (UNII: WV9CM0O67Z) BIOTIN (UNII: 6SO6U10H04) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83462-007-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/02/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075357 11/02/2023 Labeler - Eubizrival LLC (036572203) Establishment Name Address ID/FEI Business Operations Guangzhou Hanhai Trading Co., Ltd 419707381 manufacture(83462-007) , label(83462-007)