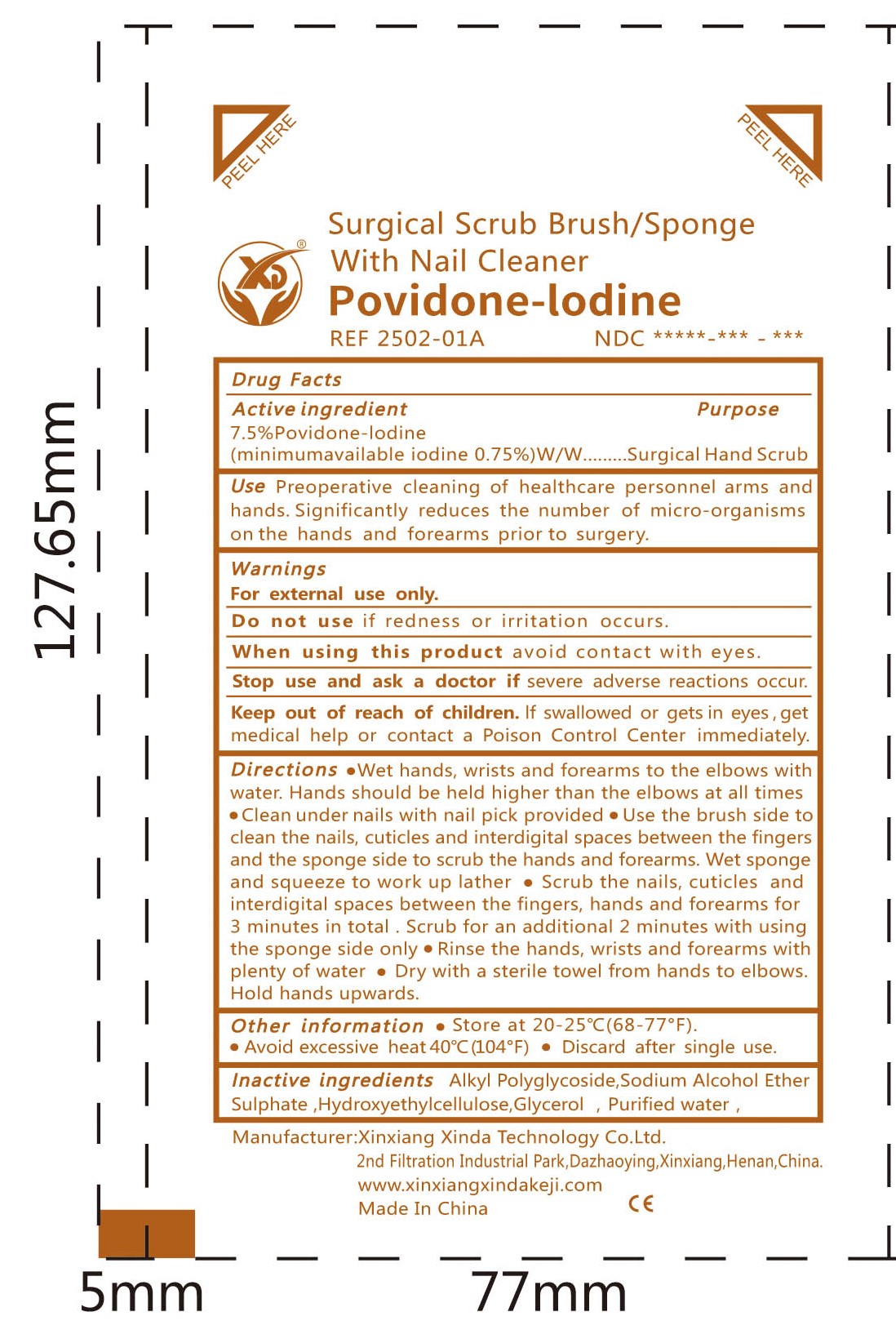
Label: SURGICAL SCRUB BRUSH/SPONGE WITH NAIL CLEANER- povidone iodine sponge
- NDC Code(s): 84128-001-01
- Packager: Xinxiang Xinda Technology Co.,Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
●Wet hands, wrists and forearms to the elbows with water. Hands should be held higher than the elbows at all times
●Clean under nails with nail pick provided · Use the brush side toclean the nails, cuticles and interdigital spaces between the fingersand the sponge side to scrub the hands and forearms. Wet sponge and squeeze to work up lather ·
●Scrub the nails, cuticles and interdigital spaces between the fingers, hands and forearms for 3 minutes in total . Scrub for an additional 2 minutes with using the sponge side only
●Rinse the hands, wrists and forearms with plenty of water
●Dry with a sterile towel from hands to elbows.Hold hands upwards. - Inactive ingredients
- Other information
- REF 2502-01A
-
INGREDIENTS AND APPEARANCE
SURGICAL SCRUB BRUSH/SPONGE WITH NAIL CLEANER
povidone iodine spongeProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84128-001 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 7.5 g in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM LAURETH-5 SULFATE (UNII: 410Q7WN1BX) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) POLYDEXTROSE (UNII: VH2XOU12IE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84128-001-01 30 in 1 BOX 02/27/2024 1 1 in 1 BAG 1 20 mL in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 02/27/2024 Labeler - Xinxiang Xinda Technology Co.,Ltd (546423962) Establishment Name Address ID/FEI Business Operations Xinxiang Xinda Technology Co.,Ltd 546423962 manufacture(84128-001)