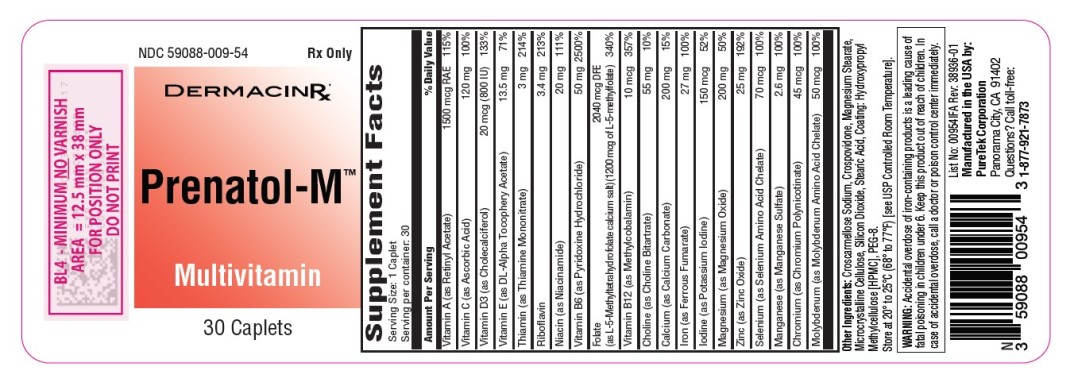
Label: PRENATOL-M- multivitamin tablet
- NDC Code(s): 59088-009-54
- Packager: PureTek Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION:
Each caplet contains:
Vitamin A (as Retinyl Acetate) ………………....… 1500 mcg RAE
Vitamin C (as Ascorbic Acid) ………………….………… 120 mg
Vitamin D3 (as Cholecalciferol).……………....... 20 mcg (800 IU)
Vitamin E (as Dl-Alpha Tocopheryl Acetate) ….......….…. 13.5 mg
Thiamin (as Thiamine Mononitrate) …………...…………… 3 mg
Riboflavin ……………………….…………………….…. 3.4 mg
Niacin (as Niacinamide) ………………..……………….… 20 mg
Vitamin B6 (as Pyridoxine Hydrochloride) …....…………... 50 mg
Folate (as L-5-Methyltetrahydrofolate calcium salt) ….... 2040 mcg DFE
(1200 mcg as L-5-Methylfolate)
Vitamin B12 (as Methylcobalamin) ……….……………... 10 mcg
Choline (as Choline Bitartrate) …….……………………… 55 mg
Calcium (as Calcium Carbonate) ……...…………………. 200 mg
Iron (as Ferrous Fumarate) ………..……………………… 27 mg
Iodine (as Potassium Iodine) …...……………………….150 mcg
Magnesium (as Magnesium Oxide) …...………………… 200 mg
Zinc (as Zinc Oxide) ………………...…………………….. 25 mg
Selenium (as Selenium Amino Acid Chelate) ……………. 70 mcg
Manganese (as Manganese Sulfate) ……………………… 2.6 mg
Chromium (as Chromium Polynicotinate) ……………….. 45 mcg
Molybdenum (as Molybdenum Amino Acid Chelate)…...… 50 mcg - Other Ingredients:
-
Indications and Usage: Prenatol-M™
Indications and Usage: Prenatol-M™ is indicated to provide vitamins and minerals to women throughout pregnancy and during the postnatal period for both lactating and non-lactating mothers, and throughout the childbearing years.
Prenatol-M™ may be beneficial in improving the nutritional status of women prior to conception.
-
Contraindications:
This product is contraindicated in patients with known hypersensitivity to any of its ingredients; also, all iron compounds are contraindicated in patients with hemosiderosis, hemochromatosis, or hemolytic anemias. Pernicious anemia is a contraindication, as folate may obscure its signs and symptoms.
-
Warnings:
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. In case of accidental overdose, call a doctor or poison control center immediately. Administration of folate alone is improper therapy for pernicious anemia and other megaloblastic anemias in which vitamin B12 is deficient.
Precautions:
Folate in doses above 0.1 mg daily may obscure pernicious anemia, in that hematologic remission can occur while neurological manifestations remain progressive. There is a potential danger in administering folate to patients with undiagnosed anemia since folic acid may obscure the diagnosis of pernicious anemia by alleviating the hematologic manifestations of the disease while allowing the neurologic complications to progress. This may result in severe nervous system damage before the correct diagnosis is made. Adequate doses of vitamin B12 may prevent, halt, or improve the neurologic changes caused by pernicious anemia. The patient’s medical conditions and consumption of other drugs, herbs, and/or supplements should be considered.
For use on the order of a licensed healthcare practitioner. Call your doctor about side effects. To report side effects, call PureTek
Corporation at 1-877-921-7873 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. - Drug Interactions:
-
Adverse Reactions:
Folate: Allergic sensitizations have been reported following both oral and parenteral administration of folate.
Ferrous Fumarate: Gastrointestinal disturbances (anorexia, nausea, diarrhea, constipation) occur occasionally but are usually mild and may subside with continuation of therapy. Although the absorption of iron is best when taken between meals, giving Prenatol-M™ after meals may control occasional gastrointestinal disturbances. Prenatol-M™ is best absorbed when taken at bedtime.
Adverse reactions have been reported with specific vitamins and minerals but generally at levels substantially higher than those contained herein. However, allergic, and idiosyncratic reactions are possible at lower levels. Iron, even at the usual recommended levels, has been associated with gastrointestinal intolerance in some patients.
-
Overdose:
Iron: signs and symptoms: Iron is toxic. Acute overdosage of iron may cause nausea and vomiting and, in severe cases, cardiovascular collapse and death. Other symptoms include pallor and cyanosis,melena, shock, drowsiness and coma. The estimated overdose of orally ingested iron is 300 mg/kg body weight. When overdoses are ingested by children, severe reactions, including fatalities, have resulted. Prenatol-M™ should be stored beyond the reach of children to prevent against accidental iron poisoning. Keep this and all other drugs out of the reach of children.
- Treatment:
- Dosage and Administration:
- How Supplied:
- Storage
- Prenatol-M™
-
INGREDIENTS AND APPEARANCE
PRENATOL-M
multivitamin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59088-009 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 20 ug FERROUS FUMARATE (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 27 mg MOLYBDENUM (UNII: 81AH48963U) (MOLYBDENUM - UNII:81AH48963U) MOLYBDENUM 50 ug ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 120 mg ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 25 mg CHOLINE BITARTRATE (UNII: 6K2W7T9V6Y) (CHOLINE - UNII:N91BDP6H0X) CHOLINE 55 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 3.4 mg MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM OXIDE 200 mg .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 13.5 mg VITAMIN A ACETATE (UNII: 3LE3D9D6OY) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 1500 ug SELENIUM (UNII: H6241UJ22B) (SELENIUM - UNII:H6241UJ22B) SELENIUM 70 ug NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 20 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 50 mg CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 200 mg CHROMIUM NICOTINATE (UNII: A150AY412V) (CHROMIC CATION - UNII:X1N4508KF1) CHROMIUM NICOTINATE 45 ug MANGANESE SULFATE (UNII: W00LYS4T26) (MANGANOUS CATION - UNII:H6EP7W5457) MANGANOUS CATION 2.6 mg POTASSIUM IODIDE (UNII: 1C4QK22F9J) (IODIDE ION - UNII:09G4I6V86Q) POTASSIUM IODIDE 150 ug METHYLCOBALAMIN (UNII: BR1SN1JS2W) (METHYLCOBALAMIN - UNII:BR1SN1JS2W) METHYLCOBALAMIN 10 ug LEVOMEFOLATE CALCIUM (UNII: A9R10K3F2F) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLATE CALCIUM 2040 ug THIAMINE MONONITRATE (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 3 mg Inactive Ingredients Ingredient Name Strength STEARIC ACID (UNII: 4ELV7Z65AP) HYPROMELLOSES (UNII: 3NXW29V3WO) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) CROSPOVIDONE (UNII: 2S7830E561) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) Product Characteristics Color brown (Beige, Speckled) Score no score Shape CAPSULE (Oblong) Size 22mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59088-009-54 30 in 1 BOTTLE; Type 0: Not a Combination Product 05/06/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/06/2024 Labeler - PureTek Corporation (785961046)