Label: CAPVAXIVE- pneumococcal 21-valent conjugate vaccine injection, solution
- NDC Code(s): 0006-4347-01, 0006-4347-02, 0006-4347-99
- Packager: Merck Sharp & Dohme LLC
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated June 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CAPVAXIVE safely and effectively. See full prescribing information for CAPVAXIVE.
CAPVAXIVE™ (Pneumococcal 21-valent Conjugate Vaccine)
Injection, for intramuscular use
Initial U.S. Approval: 2024INDICATIONS AND USAGE
CAPVAXIVE™ is a vaccine indicated for:
- active immunization for the prevention of invasive disease caused by Streptococcus pneumoniae serotypes 3, 6A, 7F, 8, 9N, 10A, 11A, 12F, 15A, 15B, 15C, 16F, 17F, 19A, 20A, 22F, 23A, 23B, 24F, 31, 33F, and 35B in individuals 18 years of age and older. (1)
- active immunization for the prevention of pneumonia caused by S. pneumoniae serotypes 3, 6A, 7F, 8, 9N, 10A, 11A, 12F, 15A, 15C, 16F, 17F, 19A, 20A, 22F, 23A, 23B, 24F, 31, 33F, and 35B in individuals 18 years of age and older. (1)
The indication for the prevention of pneumonia caused by S. pneumoniae serotypes 3, 6A, 7F, 8, 9N, 10A, 11A, 12F, 15A, 15C, 16F, 17F, 19A, 20A, 22F, 23A, 23B, 24F, 31, 33F, and 35B is approved under accelerated approval based on immune responses as measured by opsonophagocytic activity (OPA). Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial. (1)
DOSAGE AND ADMINISTRATION
For intramuscular use.
- Administer a single 0.5 mL dose. (2.1)
DOSAGE FORMS AND STRENGTHS
CAPVAXIVE is an injection. A single dose is 0.5 mL. (3)
CONTRAINDICATIONS
Severe allergic reaction (e.g., anaphylaxis) to any component of CAPVAXIVE or to diphtheria toxoid. (4)
ADVERSE REACTIONS
The most commonly reported (>10%) solicited adverse reactions:
- in individuals 18 through 49 years of age were: injection-site pain (73.1%), fatigue (36.0%), headache (27.5%), myalgia (16.4%), injection-site erythema (13.8%), and injection-site swelling (13.3%). (6.1)
- in individuals 50 years of age and older were: injection-site pain (41.2%), fatigue (19.7%), and headache (11.0%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov .
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Management of Allergic Reactions
5.2 Altered Immunocompetence
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Individuals 18 years of age and older
14.2 Concomitant Vaccination
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
CAPVAXIVE™ is indicated for:
- active immunization for the prevention of invasive disease caused by Streptococcus pneumoniae serotypes 3, 6A, 7F, 8, 9N, 10A, 11A, 12F, 15A, 15B, 15C, 16F, 17F, 19A, 20A, 22F, 23A, 23B, 24F, 31, 33F, and 35B in individuals 18 years of age and older.
- active immunization for the prevention of pneumonia caused by S. pneumoniae serotypes 3, 6A, 7F, 8, 9N, 10A, 11A, 12F, 15A, 15C, 16F, 17F, 19A, 20A, 22F, 23A, 23B, 24F, 31, 33F, and 35B in individuals 18 years of age and older.
The indication for the prevention of pneumonia caused by S. pneumoniae serotypes 3, 6A, 7F, 8, 9N, 10A, 11A, 12F, 15A, 15C, 16F, 17F, 19A, 20A, 22F, 23A, 23B, 24F, 31, 33F, and 35B is approved under accelerated approval based on immune responses as measured by opsonophagocytic activity (OPA) [see Clinical Studies (14.1)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
-
2 DOSAGE AND ADMINISTRATION
For intramuscular use.
2.2 Administration
CAPVAXIVE is a colorless, clear to opalescent solution. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if particulate matter or discoloration is observed. Administer intramuscularly.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Do not administer CAPVAXIVE to individuals with a history of a severe allergic reaction (e.g., anaphylaxis) to any component of CAPVAXIVE or to diphtheria toxoid. [See Description (11).]
- 5 WARNINGS AND PRECAUTIONS
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
The most commonly reported (>10%) solicited adverse reactions in individuals 18 through 49 years of age who received CAPVAXIVE were: injection-site pain (73.1%), fatigue (36.0%), headache (27.5%), myalgia (16.4%), injection-site erythema (13.8%), and injection-site swelling (13.3%).
The most commonly reported (>10%) solicited adverse reactions in individuals 50 years of age and older who received CAPVAXIVE were: injection-site pain (41.2%), fatigue (19.7%), and headache (11.0%).
Safety Assessment in Clinical Studies
The safety of CAPVAXIVE was assessed in four clinical studies (Studies 1-4) conducted across the Americas, Europe, and Asia Pacific, which included individuals ranging in age from 18 to 97 years. Across all 4 studies, 4,556 individuals received CAPVAXIVE and 2,021 individuals received an active comparator vaccine. Safety was monitored using an electronic Vaccination Report Card for 30 days postvaccination. Injection-site adverse reactions, systemic adverse reactions, and body temperature were solicited Day 1 through Day 5 postvaccination. Unsolicited adverse events were reported Day 1 through Day 30 postvaccination. Serious adverse events (SAEs) were reported through 6 months postvaccination in all studies.
Demographics of Individuals in Clinical Studies
Across all studies, the mean age of the individuals who were randomized and vaccinated was 53.5 years, and 57.2% were female. The racial distribution was as follows: 76.0% were White, 10.2% were Black or African American, 9.9% were Asian, and 0.5% were American Indian or Alaska Native; 20.6% were of Hispanic or Latino ethnicity. Approximately 34% of vaccinated individuals had one or more prespecified chronic medical conditions known to increase the risk of pneumococcal disease (i.e., diabetes, renal disorders, chronic heart disease, chronic liver disease, chronic lung disease including asthma, smoking, alcoholism).
Pneumococcal Vaccine-Naïve Individuals 18 years of Age and Older
In a double-blind study, Study 1 (NCT05425732), individuals 18 years of age and older who had not previously received a pneumococcal vaccine were enrolled and randomized to receive a single dose of CAPVAXIVE or Prevnar 20. The percentage of individuals 18 through 49 years of age and 50 years of age and older who reported solicited adverse reactions that occurred within 5 days postvaccination of CAPVAXIVE or Prevnar 20 is shown in Table 1. Solicited adverse reactions following administration of CAPVAXIVE lasted a median of 2 days with 81.3% of reactions lasting ≤3 days for individuals 18 through 49 years of age and a median of 1 day with 86.5% of reactions lasting ≤3 days for individuals 50 years of age and older.
Table 1: Individuals With Solicited Local and Systemic Adverse Reactions Within 5 Days Postvaccination in Pneumococcal Vaccine-Naïve Individuals 18 through 49 Years of Age and 50 Years of Age and Older – Study 1 18 through 49 Years of Age 50 Years of Age and older CAPVAXIVE
n (%)Prevnar 20
n (%)CAPVAXIVE
n (%)Prevnar 20
n (%)Mild: does not interfere with activity; Moderate: interferes with activity; Severe: prevents daily activity. - *
- Every individual is counted a single time for each applicable row and column.
- †
- Injection-site erythema, injection-site pain, injection-site swelling, fatigue, headache, and myalgia were solicited from Day 1 through Day 5 postvaccination.
- ‡
- Includes one individual with an event of missing / unknown intensity.
- §
- Pyrexia was defined as temperature ≥38.0°C (100.4°F) solicited from Day 1 through Day 5 postvaccination. Percentages are based on the number of individuals with temperature data: 18 through 49 years of age: CAPVAXIVE, n=199, Prevnar 20, n=100. 50 years of age and older: CAPVAXIVE, n=1169, Prevnar 20, n=1170.
Individuals in population* 200 100 1177 1175 Local adverse reactions† Severity Pain Any 143 (71.5) 74 (74.0) 464 (39.4) 607 (51.7) Mild 95 (47.5) 49 (49.0) 361 (30.7) 504 (42.9) Moderate 46 (23.0) 25 (25.0) 102 (8.7) 102 (8.7) Severe 2 (1.0) 0 1 (0.1) 1 (0.1) Erythema Any 31 (15.5) 13 (13.0) 64 (5.4) ‡ 74 (6.3) ‡ Mild (≤5.0 cm) 23 (11.5) 10 (10.0) 51 (4.3) 59 (5.0) Moderate (>5.0 to ≤10.0 cm) 7 (3.5) 3 (3.0) 10 (0.8) 12 (1.0) Severe (>10.0 cm) 1 (0.5) 0 2 (0.2) 2 (0.2) Swelling Any 28 (14.0) 14 (14.0) 71 (6.0) 98 (8.3) Mild (≤5.0 cm) 20 (10.0) 9 (9.0) 53 (4.5) 79 (6.7) Moderate (>5.0 to ≤10.0 cm) 7 (3.5) 5 (5.0) 15 (1.3) 17 (1.4) Severe (>10.0 cm) 1 (0.5) 0 3 (0.3) 2 (0.2) Systemic adverse reactions† Severity Fatigue Any 81 (40.5) 34 (34.0) 237 (20.1) 230 (19.6) Mild 50 (25.0) 21 (21.0) 167 (14.2) 153 (13.0) Moderate 29 (14.5) 11 (11.0) 70 (5.9) 72 (6.1) Severe 2 (1.0) 2 (2.0) 0 5 (0.4) Headache Any 59 (29.5) 24 (24.0) 135 (11.5) 152 (12.9) Mild 44 (22.0) 17 (17.0) 102 (8.7) 106 (9.0) Moderate 14 (7.0) 7 (7.0) 33 (2.8) 45 (3.8) Severe 1 (0.5) 0 0 1 (0.1) Myalgia Any 33 (16.5) 14 (14.0) 70 (5.9) 79 (6.7) Mild 15 (7.5) 9 (9.0) 40 (3.4) 42 (3.6) Moderate 15 (7.5) 4 (4.0) 30 (2.5) 36 (3.1) Severe 3 (1.5) 1 (1.0) 0 1 (0.1) Pyrexia § ≥38.0°C (100.4°F) 7 (3.5) 1 (1.0) 15 (1.3) 15 (1.3) ≥38.0°C (100.4°F) to <38.5°C (101.3°F) 3 (1.5) 0 7 (0.6) 7 (0.6) ≥38.5°C (101.3°F) to <39.0°C (102.2°F) 2 (1.0) 0 6 (0.5) 5 (0.4) ≥39.0°C (102.2°F) 2 (1.0) 1 (1.0) 2 (0.2) 3 (0.3) In Study 2 (NCT05464420), individuals 18 through 49 years of age who had not previously received a pneumococcal vaccine were enrolled and randomized to receive a single dose of CAPVAXIVE or PNEUMOVAX 23. The percentage of individuals 18 through 49 years of age with solicited adverse reactions that occurred within 5 days postvaccination of CAPVAXIVE or PNEUMOVAX 23 is shown in Table 2.
Table 2: Individuals with Solicited Local and Systemic Adverse Reactions Within 5 Days Postvaccination in Pneumococcal Vaccine-Naïve Individuals 18 through 49 Years of Age – Study 2 CAPVAXIVE
n (%)PNEUMOVAX 23
n (%)Mild: does not interfere with activity; Moderate: interferes with activity; Severe: prevents daily activity - *
- Every individual is counted a single time for each applicable row and column.
- †
- Injection-site erythema, injection-site pain, injection-site swelling, fatigue, headache, and myalgia were solicited from Day 1 through Day 5 postvaccination.
- ‡
- Pyrexia was defined as temperature ≥38.0°C (100.4°F) solicited from Day 1 through Day 5 postvaccination. Percentages are based on the number of individuals with temperature data: CAPVAXIVE, n=1,606; PNEUMOVAX 23, n=541.
Individuals in population* 1,616 541 Local adverse reactions† Severity Pain Any 1,184 (73.3) 328 (60.6) Mild 759 (47.0) 234 (43.3) Moderate 395 (24.4) 86 (15.9) Severe 30 (1.9) 8 (1.5) Erythema Any 219 (13.6) 41 (7.6) Mild (≤5.0 cm) 143 (8.8) 30 (5.5) Moderate (>5.0 to ≤10.0 cm) 57 (3.5) 8 (1.5) Severe (>10.0 cm) 19 (1.2) 3 (0.6) Swelling Any 213 (13.2) 41 (7.6) Mild (≤5.0 cm) 148 (9.2) 29 (5.4) Moderate (>5.0 to ≤10.0 cm) 55 (3.4) 10 (1.8) Severe (>10.0 cm) 10 (0.6) 2 (0.4) Systemic adverse reactions† Severity Fatigue Any 573 (35.5) 184 (34.0) Mild 338 (20.9) 119 (22.0) Moderate 201 (12.4) 60 (11.1) Severe 34 (2.1) 5 (0.9) Headache Any 440 (27.2) 116 (21.4) Mild 275 (17.0) 70 (12.9) Moderate 151 (9.3) 43 (7.9) Severe 14 (0.9) 3 (0.6) Myalgia Any 264 (16.3) 47 (8.7) Mild 146 (9.0) 33 (6.1) Moderate 103 (6.4) 12 (2.2) Severe 15 (0.9) 2 (0.4) Pyrexia‡ ≥38.0°C (100.4°F) 48 (3.0) 12 (2.2) ≥38.0°C (100.4°F) to <38.5°C (101.3°F) 31 (1.9) 4 (0.7) ≥38.5°C (101.3°F) to <39.0°C (102.2°F) 11 (0.7) 2 (0.4) ≥39.0°C (102.2°F) 6 (0.4) 6 (1.1) Individuals 50 Years of Age and Older Who Previously Received Pneumococcal Vaccines
Study 3 (NCT05420961) enrolled individuals 50 years of age and older who had previously received a pneumococcal vaccine at least 1 year prior to enrollment. Participants were enrolled into 1 of 3 cohorts based on their pneumococcal vaccination history (cohort 1: PNEUMOVAX 23, cohort 2: Prevnar 13, or cohort 3: PNEUMOVAX 23 followed by or preceded by Prevnar 13, PNEUMOVAX 23 preceded by VAXNEUVANCE, or VAXNEUVANCE alone). Participants in cohort 1 were randomized to receive CAPVAXIVE or VAXNEUVANCE, participants in cohort 2 were randomized to receive CAPVAXIVE or PNEUMOVAX 23, and participants in cohort 3 received CAPVAXIVE. The percentage of individuals with solicited adverse reactions that occurred within 5 days postvaccination of CAPVAXIVE or active comparator is shown in Table 3.
Table 3: Individuals with Solicited Local and Systemic Adverse Reactions Within 5 Days Postvaccination in Individuals 50 Years of Age and Older with Prior Pneumococcal Vaccination – Study 3 Cohort 1* Cohort 2† Cohort 3‡ CAPVAXIVE
n (%)VAXNEUVANCE
n (%)CAPVAXIVE
n (%)PNEUMOVAX 23
n (%)CAPVAXIVE
n (%)Mild: does not interfere with activity; Moderate: interferes with activity; Severe: prevents daily activity - *
- Cohort 1 prior vaccination with PNEUMOVAX 23
- †
- Cohort 2 prior vaccination with Prevnar 13
- ‡
- Cohort 3 prior vaccination with Prevnar 13+PNEUMOVAX 23 (n=45), or VAXNEUVANCE+PNEUMOVAX 23 (n=5), or PNEUMOVAX 23+Prevnar 13 (n=54), or VAXNEUVANCE (n=1) or Prevnar 20 (n=0)
- §
- Every individual is counted a single time for each applicable row and for each column.
- ¶
- Injection-site erythema, injection-site pain, injection-site swelling, fatigue, headache, and myalgia were solicited from Day 1 through Day 5 postvaccination.
- #
- Pyrexia was defined as temperature ≥38.0°C (100.4°F) solicited from Day 1 through Day 5 postvaccination.
Individuals in population§ 230 117 174 85 105 Local adverse reactions¶ Severity Pain Any 82 (35.7) 51 (43.6) 72 (41.4) 40 (47.1) 46 (43.8) Mild 65 (28.3) 43 (36.8) 52 (29.9) 30 (35.3) 37 (35.2) Moderate 16 (7.0) 8 (6.8) 20 (11.5) 10 (11.8) 9 (8.6) Severe 1 (0.4) 0 0 0 0 Erythema Any 17 (7.4) 9 (7.7) 13 (7.5) 8 (9.4) 8 (7.6) Mild (≤5.0 cm) 10 (4.3) 6 (5.1) 5 (2.9) 2 (2.4) 4 (3.8) Moderate (>5.0 to ≤10.0 cm) 5 (2.2) 2 (1.7) 6 (3.4) 6 (7.1) 3 (2.9) Severe (>10.0 cm) 2 (0.9) 1 (0.9) 2 (1.1) 0 1 (1.0) Swelling Any 19 (8.3) 10 (8.5) 8 (4.6) 14 (16.5) 11 (10.5) Mild (≤5.0 cm) 15 (6.5) 9 (7.7) 6 (3.4) 7 (8.2) 6 (5.7) Moderate (>5.0 to ≤10.0 cm) 4 (1.7) 1 (0.9) 2 (1.1) 7 (8.2) 4 (3.8) Severe (>10.0 cm) 0 0 0 0 1 (1.0) Systemic adverse reactions¶ Severity Fatigue Any 33 (14.3) 20 (17.1) 33 (19.0) 11 (12.9) 23 (21.9) Mild 25 (10.9) 11 (9.4) 24 (13.8) 6 (7.1) 19 (18.1) Moderate 8 (3.5) 9 (7.7) 8 (4.6) 5 (5.9) 4 (3.8) Severe 0 0 1 (0.6) 0 0 Headache Any 16 (7.0) 11 (9.4) 18 (10.3) 10 (11.8) 9 (8.6) Mild 10 (4.3) 9 (7.7) 10 (5.7) 7 (8.2) 9 (8.6) Moderate 5 (2.2) 2 (1.7) 8 (4.6) 3 (3.5) 0 Severe 1 (0.4) 0 0 0 0 Myalgia Any 17 (7.4) 3 (2.6) 17 (9.8) 8 (9.4) 9 (8.6) Mild 9 (3.9) 2 (1.7) 7 (4.0) 4 (4.7) 7 (6.7) Moderate 8 (3.5) 1 (0.9) 9 (5.2) 4 (4.7) 2 (1.9) Severe 0 0 1 (0.6) 0 0 Pyrexia # ≥38.0°C (100.4°F) 4 (1.7) 3 (2.6) 5 (2.9) 1 (1.2) 0 ≥38.0°C (100.4°F) to <38.5°C (101.3°F) 2 (0.9) 0 1 (0.6) 0 0 ≥38.5°C (101.3°F) to <39.0°C (102.2°F) 2 (0.9) 2 (1.7) 2 (1.1) 1 (1.2) 0 ≥39.0°C (102.2°F) 0 1 (0.9) 2 (1.1) 0 0 Safety with Concomitant Influenza Vaccine Administration
In Study 4 (NCT05526716), individuals 50 years of age and older with or without a history of prior pneumococcal vaccination were enrolled and randomized to receive either CAPVAXIVE and quadrivalent influenza vaccine [Fluzone Quadrivalent, (QIV)] concomitantly followed by placebo 30 days later (concomitant group), or QIV and placebo concomitantly followed by CAPVAXIVE 30 days later (sequential group).
In Study 4, the rates and severity of solicited systemic adverse reactions and solicited local adverse reactions at the CAPVAXIVE injection site were similar when CAPVAXIVE was administered with or without inactivated QIV.
Serious Adverse Events
Across studies 1-4, the proportion of individuals reporting 1 or more SAEs within 1-month postvaccination was 0.3% in individuals vaccinated with CAPVAXIVE (n=14) and 0.3% in individuals vaccinated with an active comparator (n=7). The proportion of individuals reporting 1 or more SAEs within 6 months postvaccination was 1.4% in individuals vaccinated with CAPVAXIVE (n=56) and 2.0% in individuals vaccinated with an active comparator (n=40). There were no notable patterns or imbalances between vaccine groups for SAEs. Two individuals who received CAPVAXIVE had SAEs considered related to vaccination. One individual experienced an acute allergic reaction of bronchospasm (Grade 3, required medical intervention) which occurred within 30 minutes postvaccination; one individual experienced injection-site cellulitis (Grade 4, required hospitalization) on Day 6 postvaccination.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
There are no adequate and well-controlled studies of CAPVAXIVE in pregnant individuals. Data on CAPVAXIVE administered to pregnant individuals are insufficient to inform vaccine-associated risks in pregnancy.
A developmental toxicity study has been performed in female rats administered 0.25 mL of a conjugated polysaccharide vaccine formulation on four occasions: twice prior to mating, once during gestation, and once during lactation. This study revealed no adverse effects on fetal or preweaning development. [see Animal Data below].
Data
In a developmental toxicity study, female rats were administered 0.25 mL of a conjugated polysaccharide vaccine formulation containing the same conjugated polysaccharides as in CAPVAXIVE. Animals received 42 mcg polysaccharide per dose (a full human dose of CAPVAXIVE contains 84 mcg polysaccharide/dose) by intramuscular injection on four occasions: 28 and 7 days prior to mating, on gestation day 6, and on lactation day 7. There were no embryofetal deaths or fetal malformations, and no adverse effects on female fertility and preweaning development were observed.
8.2 Lactation
Risk Summary
Human data are not available to assess the impact of CAPVAXIVE on milk production, its presence in breast milk, or its effects on the breastfed child. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for CAPVAXIVE and any potential adverse effects on the breastfed child from CAPVAXIVE or from the underlying maternal condition. For preventive vaccines, the underlying condition is susceptibility to disease prevented by the vaccine.
8.4 Pediatric Use
The safety and effectiveness of CAPVAXIVE in individuals younger than 18 years of age have not been established.
8.5 Geriatric Use
Across studies 1-4, of the 4,556 individuals who received CAPVAXIVE, 1,487 individuals (32.6%) were 65 years of age and older, and 339 individuals (7.4%) were 75 years of age and older. In Study 1, of the 1,379 individuals who received CAPVAXIVE, 590 individuals (42.8%) were 65 years of age and older, and 126 individuals (9.1%) were 75 years of age and older. No clinically meaningful differences in safety of CAPVAXIVE were observed between these individuals and individuals less than 65 years of age. The opsonophagocytic activity (OPA) responses in individuals 65 years of age and older were generally lower than those observed in individuals less than 65 years of age.
-
11 DESCRIPTION
CAPVAXIVE (Pneumococcal 21-valent Conjugate Vaccine) is an injection for intramuscular use. CAPVAXIVE is a sterile solution of purified capsular polysaccharides from S. pneumoniae serotypes 3, 6A, 7F, 8, 9N, 10A, 11A, 12F, 15A, 15B (de-O-acetylated prior to conjugation), 16F, 17F, 19A, 20A, 22F, 23A, 23B, 24F, 31, 33F, and 35B individually conjugated to CRM197 carrier protein. CRM197 is a nontoxic mutant of diphtheria toxin (originating from Corynebacterium diphtheriae C7) expressed recombinantly in Pseudomonas fluorescens.
Each S. pneumoniae serotype is grown separately in media containing yeast extract, dextrose, salts, and soy peptone. The pneumococcal bacteria are inactivated after growth by addition of phenol to the culture media. Subsequently, each polysaccharide is purified to produce a powder using a series of chemical and physical methods. Serotype 15B polysaccharide is de-O-acetylated (deOAc 15B). The purified polysaccharides are chemically activated. Recombinant P. fluorescens expressing CRM197 is grown in a glycerol-based, chemically-defined salt medium. The CRM197 is then purified by chromatography and ultrafiltration. Each polysaccharide is individually conjugated to CRM197 carrier protein to create 21 individual conjugates. The final vaccine is prepared by blending the 21 conjugated polysaccharides in a final buffer containing histidine, polysorbate 20, and sodium chloride.
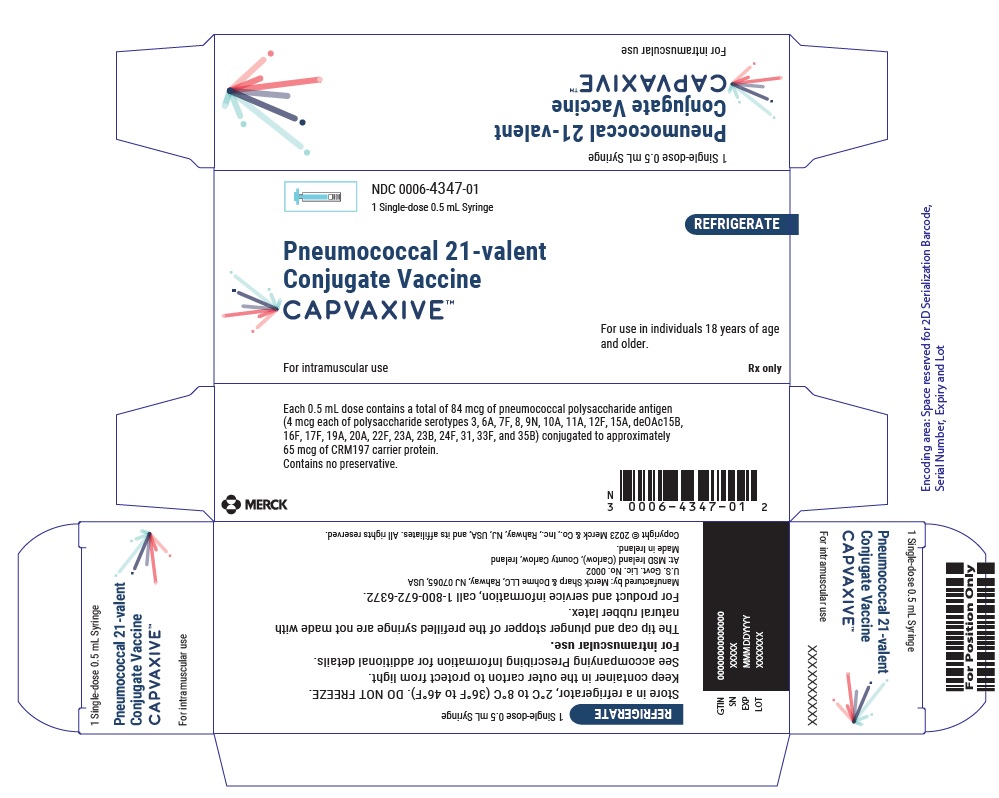
Each 0.5 mL dose contains a total of 84 mcg of pneumococcal polysaccharide antigen (4 mcg each of polysaccharide serotypes 3, 6A, 7F, 8, 9N, 10A, 11A, 12F, 15A, 15B (deOAc 15B), 16F, 17F, 19A, 20A, 22F, 23A, 23B, 24F, 31, 33F, and 35B) conjugated to approximately 65 mcg of CRM197 carrier protein, 1.55 mg L-histidine, 0.50 mg of polysorbate 20, 4.49 mg sodium chloride, and water for injection.
CAPVAXIVE does not contain any preservatives.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Protection against invasive pneumococcal disease is conferred mainly by opsonophagocytic killing of S. pneumoniae. CAPVAXIVE induces OPA against 22 S. pneumoniae serotypes. The de-O-acetylated polysaccharide from serotype 15B has a molecular structure similar to the polysaccharide from serotype 15C and induces OPA to serotype 15C. The deOAc15B also induces cross-reactive OPA against serotype 15B. An OPA titer that is predictive of protection against invasive pneumococcal disease or pneumococcal pneumonia has not been established.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
For studies 1-4, immunogenicity was assessed by serotype-specific opsonophagocytic activity (OPA) responses at 1-month postvaccination. The primary immunogenicity endpoints included OPA geometric mean titers (GMTs) and the proportion of individuals who achieved ≥4-fold rise in OPA responses from prevaccination to 1-month postvaccination.
14.1 Individuals 18 years of age and older
The effectiveness of CAPVAXIVE in individuals 18 years of age and older for the prevention of invasive disease caused by S. pneumoniae serotypes 3, 6A, 7F, 8, 9N, 10A, 11A, 12F, 15A, 15B, 15C, 16F, 17F, 19A, 20A, 22F, 23A, 23B, 24F, 31, 33F and 35B and for the prevention of pneumonia caused by S. pneumoniae serotypes 3, 6A, 7F, 8, 9N, 10A, 11A, 12F, 15A, 15C, 16F, 17F, 19A, 20A, 22F, 23A, 23B, 24F, 31, 33F, and 35B was demonstrated based on comparative immunogenicity to a licensed pneumococcal vaccine (Prevnar 20).
Pneumococcal Vaccine-Naïve Individuals 50 years of age and older
In Study 1, 2,362 pneumococcal vaccine-naïve individuals 50 years of age and older were randomized to receive either CAPVAXIVE or Prevnar 20. [see Adverse Reactions (6.1).]
Table 4 summarizes the 21 serotype-specific OPA geometric mean antibody titers (GMTs) at 30 days postvaccination. The study demonstrated that CAPVAXIVE is noninferior to Prevnar 20 for the 10 shared serotype polysaccharides and induces statistically significantly greater OPA GMTs compared to Prevnar 20 for 10 of 11 serotype polysaccharides unique to CAPVAXIVE. Serotype 15C did not meet the criterion for statistical significance.
Table 5 summarizes the proportion of individuals who achieved a ≥4-fold rise from prevaccination to 1-month postvaccination for OPA responses. For 10 of 11 serotype polysaccharides unique to CAPVAXIVE, CAPVAXIVE induced statistically significantly greater OPA responses compared to Prevnar 20. Serotype 15C did not meet the criterion for statistical significance.
Table 4: Serotype-Specific OPA GMTs in Pneumococcal Vaccine-Naïve Individuals 50 Years of Age and Older (Study 1) Pneumococcal
SerotypeCAPVAXIVE
(N = 1179)Prevnar 20
(N = 1177)GMT Ratio*
(CAPVAXIVE/Prevnar 20)
(95% CI)*n GMT* n GMT* N=Number of individuals randomized and vaccinated; n=Number of individuals contributing to the analysis. - *
- GMTs, GMT ratio, and 95% CI were estimated from a constrained Longitudinal Data Analysis model.
- †
- Non-inferiority for the serotypes common to CAPVAXIVE and Prevnar 20 was based on the lower bound of the 2-sided 95% CI for the estimated GMT ratio (CAPVAXIVE/Prevnar 20) being >0.5.
- ‡
- Statistically significantly greater OPA responses for the serotypes unique to CAPVAXIVE compared to Prevnar 20 were based on the lower bound of the 2-sided 95% CI for the estimated GMT ratio (CAPVAXIVE/Prevnar 20) being >2.0.
10 Common Serotypes† 3 1154 274.0 1161 176.7 1.55 (1.40, 1.72) 6A 1148 2302.0 1153 2972.5 0.77 (0.68, 0.88) 7F 1152 3637.4 1158 3429.9 1.06 (0.95, 1.18) 8 1155 2501.3 1158 1811.1 1.38 (1.25, 1.53) 10A 1161 3893.4 1159 4678.0 0.83 (0.75, 0.93) 11A 1145 3232.6 1150 2092.8 1.54 (1.39, 1.72) 12F 1160 2641.2 1161 2499.6 1.06 (0.92, 1.21) 19A 1159 2136.1 1162 2817.8 0.76 (0.69, 0.84) 22F 1147 3874.5 1154 4770.1 0.81 (0.72, 0.92) 33F 1154 13558.9 1157 11742.1 1.15 (1.01, 1.32) 11 Serotypes Unique to CAPVAXIVE ‡ 9N 1147 7470.7 1150 1640.4 4.55 (4.12, 5.04) 15A 1107 5237.2 1102 1589.0 3.30 (2.91, 3.74) 15C 1153 4216.2 1158 2072.3 2.03 (1.77, 2.34) 16F 1151 4868.2 1153 846.3 5.75 (5.16, 6.41) 17F 1148 7764.9 1156 460.4 16.86 (14.90, 19.09) 20A 1161 6099.2 1155 631.1 9.66 (8.66, 10.79) 23A 1132 3737.2 1104 461.5 8.10 (6.86, 9.55) 23B 1160 1082.5 1160 107.3 10.09 (8.48, 12.00) 24F 1153 2728.6 1130 70.5 38.71 (33.87, 44.25) 31 1153 3132.5 1154 144.4 21.69 (18.68, 25.18) 35B 1153 8527.8 1159 1383.0 6.17 (5.59, 6.80) Table 5: Pneumococcal Vaccine-Naïve Individuals 50 years of Age and Older With a ≥4-Fold Rise in OPA Responses for Serotypes Unique to CAPVAXIVE (Study 1) Pneumococcal
SerotypeCAPVAXIVE
(N=1179)Prevnar 20
(N=1177)Percentage Point Difference
(CAPVAXIVE – Prevnar 20)Observed Response
Percentage (m/n)Observed Response
Percentage (m/n)Estimate (95% CI)*,† N=Number of individuals randomized and vaccinated; m=Number of individuals with the indicated response; n=Number of individuals contributing to the analysis - *
- Estimated difference and CI were based on the stratified Miettinen & Nurminen method.
- †
- Statistically significantly greater OPA responses were based on the lower bound of the 2-sided 95% CI of the differences (CAPVAXIVE – Prevnar 20) between the percentages of individuals with a ≥4-fold rise from prevaccination to 1-month postvaccination being >10 percentage points.
9N 64.7 (595/920) 19.9 (195/978) 44.7 (40.7, 48.6) 15A 66.7 (462/693) 35.8 (253/706) 30.9 (25.8, 35.8) 15C 83.4 (794/952) 74.2 (695/937) 9.2 (5.6, 12.9) 16F 71.9 (654/910) 20.8 (200/961) 51.1 (47.1, 54.9) 17F 75.8 (653/862) 9.5 (90/952) 66.3 (62.8, 69.6) 20A 67.3 (675/1003) 9.6 (97/1011) 57.7 (54.2, 61.1) 23A 78.9 (598/758) 36.8 (270/734) 42.2 (37.6, 46.6) 23B 85.5 (873/1021) 49.6 (506/1021) 35.9 (32.1, 39.6) 24F 80.5 (745/925) 6.3 (55/872) 74.2 (71.1, 77.1) 31 76.5 (698/912) 17.9 (171/954) 58.6 (54.8, 62.1) 35B 60.0 (550/917) 6.8 (67/988) 53.2 (49.6, 56.6) In Study 1, 64.7% of individuals 50 years of age and older, who received CAPVAXIVE, had ≥4-fold rise in cross-reactive OPA titers for serotype 15B, which met the prespecified success criterion (lower bound of the 2-sided 95% CI of the proportion of individuals with a ≥4-fold rise in OPA responses is >50%). In a descriptive analysis, the S. pneumoniae serotype 15B OPA GMT was 4,400.6 following administration of CAPVAXIVE, and 4,640.0 following administration of Prevnar 20, with a GMT ratio of 0.95 (95% CI: 0.84, 1.07).
Pneumococcal Vaccine-Naïve Individuals 18 through 49 Years of Age
In Study 1, pneumococcal vaccine-naïve individuals 18 through 49 years of age were randomized in a 2:1 ratio to receive CAPVAXIVE or Prevnar 20. [See Adverse Reactions (6.1).]
Effectiveness of CAPVAXIVE in individuals 18 through 49 years of age was assessed by a comparison of the OPA responses induced by CAPVAXIVE in this age group to the OPA responses of individuals 50 through 64 years of age. The OPA responses of individuals 18 through 49 years of age to each of 22 S. pneumoniae serotypes met the criteria for immunobridging as the lower bound of the 2-sided 95% CI for the GMT ratio for each serotype was >0.5 (see Table 6). The S. pneumoniae serotype 15B cross-reactive OPA GMT was 10,976.7 following administration of CAPVAXIVE in individuals 18 through 49 years of age and 5,438.9 following administration of CAPVAXIVE in individuals 50 through 64 years of age, with a GMT ratio of 2.02 (95% CI: 1.57, 2.60).
Table 6: Comparison of Serotype-Specific OPA GMTs in Pneumococcal Vaccine-Naïve Individuals 18 through 49 Years of Age to 50 through 64 Years of Age Who Received CAPVAXIVE (Study 1) Pneumococcal
Serotype18 through 49 years
(N = 200)50 through 64 years
(N = 589)GMT Ratio*,†
(18 through 49 years / 50
through 64 years)
(95% CI)*n GMT* n GMT N=Number of individuals randomized and vaccinated; n=Number of individuals contributing to the analysis. 3 194 308.6 572 282.7 1.09 (0.90, 1.33) 6A 196 5289.6 569 2572.9 2.06 (1.61, 2.62) 7F 198 6447.2 571 4278.8 1.51 (1.23, 1.84) 8 197 4516.0 571 3004.7 1.50 (1.26, 1.79) 9N 197 17283.2 570 8791.4 1.97 (1.59, 2.43) 10A 197 6808.1 575 4382.6 1.55 (1.26, 1.92) 11A 196 5871.6 564 3785.8 1.55 (1.26, 1.91) 12F 196 6150.4 574 3561.2 1.73 (1.37, 2.17) 15A 184 11319.2 550 5901.2 1.92 (1.55, 2.37) 15C 195 10194.0 570 5708.0 1.79 (1.36, 2.35) 16F 193 8877.0 571 5720.0 1.55 (1.26, 1.91) 17F 194 16070.6 568 10068.0 1.60 (1.26, 2.02) 19A 198 2773.2 574 2374.6 1.17 (0.97, 1.40) 20A 197 13150.0 575 7562.7 1.74 (1.39, 2.18) 22F 198 9299.6 568 4683.6 1.99 (1.58, 2.49) 23A 192 8848.7 561 4739.5 1.87 (1.43, 2.44) 23B 198 2140.1 575 1420.9 1.51 (1.11, 2.04) 24F 197 4137.6 570 3047.2 1.36 (1.10, 1.67) 31 195 8005.6 570 3820.7 2.10 (1.63, 2.69) 33F 197 34805.5 570 17607.4 1.98 (1.52, 2.57) 35B 198 13933.4 573 9053.9 1.54 (1.26, 1.87) Individuals 50 years of age and older with Prior Pneumococcal Vaccination
Study 3, a descriptive Phase 3 study, enrolled individuals 50 years of age and older who were previously vaccinated with other pneumococcal vaccines at least 1 year prior to study entry. Participants were enrolled into 1 of 3 cohorts based on their pneumococcal vaccination history (cohort 1: PNEUMOVAX 23, cohort 2: Prevnar 13, or cohort 3: PNEUMOVAX 23 followed by or preceded by Prevnar 13, PNEUMOVAX 23 preceded by VAXNEUVANCE, or VAXNEUVANCE alone).
Participants in cohort 1 were randomized to receive CAPVAXIVE (n=231) or VAXNEUVANCE (n=119), participants in cohort 2 were randomized to receive CAPVAXIVE (n=176) or PNEUMOVAX 23 (n=85), and participants in cohort 3 were allocated to receive CAPVAXIVE (n=106).
In each of the 3 cohorts, serotype-specific OPA GMTs and the proportion of individuals with ≥4-fold rise in OPA responses from baseline to 1-month postvaccination were assessed. In Cohort 1, CAPVAXIVE elicited OPA responses that were comparable to VAXNEUVANCE for the 6 common serotypes, and higher for the 15 unique serotypes and serotype 15B. In Cohort 2, CAPVAXIVE elicited OPA responses comparable to PNEUMOVAX 23 for the 12 common serotypes and serotype 15B, and higher for the 9 unique serotypes. OPA responses to CAPVAXIVE were similar across the 3 cohorts of participants who previously received one or more pneumococcal vaccines.
14.2 Concomitant Vaccination
In a double-blind study (Study 4), 1,080 individuals 50 years of age and older, with or without a history of prior pneumococcal vaccination, were randomized in a 1:1 ratio. One vaccination group received CAPVAXIVE and QIV concomitantly, followed by placebo 30 days later (concomitant group). A second vaccination group received QIV and placebo concomitantly, followed by CAPVAXIVE 30 days later (sequential group). Antibody responses were assessed 1-month postvaccination.
The OPA responses to CAPVAXIVE administered concomitantly with QIV were non-inferior to the OPA responses to CAPVAXIVE administered sequentially after QIV for 20 of 21 serotypes [lower bound of the 2-sided 95% CI of the GMT ratio (concomitant group/sequential group) was >0.5]; the non-inferiority was not met for serotype 23B [lower bound of the 2-sided 95% CI of the GMT ratio (concomitant group/sequential group) was 0.44]. The OPA response to serotype 15B was not assessed for non-inferiority. In a descriptive analysis, the OPA GMT in the concomitant group was 3,438.7 and in the sequential group was 4,440.5, with a GMT ratio of 0.77 (95% CI: 0.64, 0.94). The influenza strain-specific hemagglutination inhibition (HAI) responses to QIV administered concomitantly with CAPVAXIVE were non-inferior to the HAI responses to QIV administered alone for 3 of 4 influenza strains [lower bound of the 2-sided 95% CIs for HAI GMT ratios (concomitant group/sequential group) was >0.67 (non-inferiority margin); the lower bound was 0.67 for the A/H3N2 influenza strain].
-
16 HOW SUPPLIED/STORAGE AND HANDLING
CAPVAXIVE is supplied as follows:
NDC 0006-4347-01: Carton of one single-dose prefilled Luer Lock syringe with tip cap, containing 1 dose of 0.5 mL (NDC 0006-4347-99).
NDC 0006-4347-02: Carton of ten single-dose prefilled Luer Lock syringes with tip caps, each syringe containing 1 dose of 0.5 mL (NDC 0006-4347-99).
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
- Inform the patient of the benefits and risks associated with vaccination with CAPVAXIVE.
- Inform the patient that vaccination with CAPVAXIVE may not protect all vaccine recipients.
- Instruct the patient to report any adverse reactions to their healthcare provider or to the vaccine manufacturer or the U.S. Department of Health and Human Services through the Vaccine Adverse Event Reporting System (VAERS), 1-800-822-7967, or report online at www.vaers.hhs.gov.
-
SPL UNCLASSIFIED SECTION
Manufactured by: Merck Sharp & Dohme LLC
Rahway, NJ 07065, USAU.S. license number 0002
For patent information: www.msd.com/research/patent
The trademarks depicted herein are owned by their respective companies.
Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.uspi-v116-i-2406r000
-
PATIENT PACKAGE INSERT
Patient Information
CAPVAXIVE™ (pronounced “Cap-VACKS-iv”)
(Pneumococcal 21-valent Conjugate Vaccine)This Patient Information has been approved by the U.S. Food and Drug Administration Issued: 06/2024 Before you get CAPVAXIVE, read this information sheet and be sure you understand it. If you have questions or experience any side effects, talk to your healthcare provider. This information does not take the place of talking about CAPVAXIVE with your healthcare provider. Your healthcare provider will decide if CAPVAXIVE is right for you. What is CAPVAXIVE? - CAPVAXIVE is a vaccine for individuals 18 years of age and older to help protect against invasive pneumococcal disease and pneumonia caused by certain types of bacteria called pneumococcus.
- These bacteria can cause many types of illnesses, which can be severe, such as infections in:
- your lungs (pneumonia)
- the area around your brain and spinal cord (meningitis)
- your blood (bacteremia)
- You cannot get pneumococcal disease from CAPVAXIVE.
- CAPVAXIVE might not protect everyone who gets the vaccine.
Who should not get CAPVAXIVE?
Do not get CAPVAXIVE if you had an allergic reaction to any of its ingredients, including diphtheria toxoid. (See the list of ingredients at the end of this information sheet.)What should I tell my healthcare provider before getting CAPVAXIVE?
Tell your healthcare provider if you:- have had an allergic reaction to any vaccine.
- are taking any medications or treatments that might weaken the immune system (like immunosuppressants or steroids).
- have a weak immune system (which means your body is less able to fight off infections).
- are pregnant or planning to become pregnant.
- are breastfeeding or plan to breastfeed.
How is CAPVAXIVE given? - You will get an injection into the muscle (usually in your upper arm).
- You will get one dose of the vaccine.
What are the possible side effects of CAPVAXIVE?
The most common side effects of CAPVAXIVE are:- Pain, redness, or swelling where you got the injection
- Feeling tired
- Headache
- Muscle aches
- Fever
Tell your healthcare provider about these side effects or any unusual symptoms that develop after you get this vaccine. Get medical care right away if you have symptoms of an allergic reaction, which may include:- Wheezing or trouble breathing
- Swelling of the face, lips, or tongue
- Hives
- Rash
You may also report any side effects to Merck Sharp & Dohme LLC at 1-877-888-4231 or directly to Vaccine Adverse Event Reporting System (VAERS). The VAERS toll-free number is 1-800-822-7967 or report online to www.vaers.hhs.gov.Where can I get more information about CAPVAXIVE?
You can ask your pharmacist or healthcare provider for more information about CAPVAXIVE.What are the ingredients in CAPVAXIVE?
Active ingredient(s): Bacterial sugars from 21 types of pneumococcus; each linked to a protein (CRM197). The sugars from these bacteria and the protein are not alive and do not cause disease.
Inactive ingredient(s): L-histidine, polysorbate 20, sodium chloride, water.
CAPVAXIVE does not have any preservatives.
The tip cap and plunger stopper of the prefilled syringe are not made with natural rubber latex.Manufactured by: Merck Sharp & Dohme LLC, Rahway, NJ 07065, USA For patent information: www.msd.com/research/patent. The trademarks depicted herein are owned by their respective companies. Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates. All rights reserved. usppi-v116-i -2406r000 - PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe carton
- PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe carton
-
INGREDIENTS AND APPEARANCE
CAPVAXIVE
pneumococcal 21-valent conjugate vaccine injection, solutionProduct Information Product Type VACCINE Item Code (Source) NDC:0006-4347 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STREPTOCOCCUS PNEUMONIAE TYPE 3 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: 2VF3V7175U) (STREPTOCOCCUS PNEUMONIAE TYPE 3 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:2VF3V7175U) STREPTOCOCCUS PNEUMONIAE TYPE 3 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 6A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: Z9HK08690W) (STREPTOCOCCUS PNEUMONIAE TYPE 6A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:Z9HK08690W) STREPTOCOCCUS PNEUMONIAE TYPE 6A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 7F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: 0K0S2P98ZJ) (STREPTOCOCCUS PNEUMONIAE TYPE 7F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:0K0S2P98ZJ) STREPTOCOCCUS PNEUMONIAE TYPE 7F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 8 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: ADR2S9OIF2) (STREPTOCOCCUS PNEUMONIAE TYPE 8 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:ADR2S9OIF2) STREPTOCOCCUS PNEUMONIAE TYPE 8 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 9N CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: XI7GQF2NHD) (STREPTOCOCCUS PNEUMONIAE TYPE 9N CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:XI7GQF2NHD) STREPTOCOCCUS PNEUMONIAE TYPE 9N CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 10A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: N47C1SHV0F) (STREPTOCOCCUS PNEUMONIAE TYPE 10A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:N47C1SHV0F) STREPTOCOCCUS PNEUMONIAE TYPE 10A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 11A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: RDA5AKV23D) (STREPTOCOCCUS PNEUMONIAE TYPE 11A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:RDA5AKV23D) STREPTOCOCCUS PNEUMONIAE TYPE 11A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 12F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: T6O227OX7Q) (STREPTOCOCCUS PNEUMONIAE TYPE 12F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:T6O227OX7Q) STREPTOCOCCUS PNEUMONIAE TYPE 12F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 15A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: X2XG7TF9VV) (STREPTOCOCCUS PNEUMONIAE TYPE 15A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:X2XG7TF9VV) STREPTOCOCCUS PNEUMONIAE TYPE 15A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE DEOAC15B CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: ZN8Z5RQ6RC) (STREPTOCOCCUS PNEUMONIAE TYPE DEOAC15B CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:ZN8Z5RQ6RC) STREPTOCOCCUS PNEUMONIAE TYPE DEOAC15B CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 16F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: CFR26X8KJY) (STREPTOCOCCUS PNEUMONIAE TYPE 16F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:CFR26X8KJY) STREPTOCOCCUS PNEUMONIAE TYPE 16F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 17F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: DMY47L4YVQ) (STREPTOCOCCUS PNEUMONIAE TYPE 17F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:DMY47L4YVQ) STREPTOCOCCUS PNEUMONIAE TYPE 17F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 19A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: B970MQT365) (STREPTOCOCCUS PNEUMONIAE TYPE 19A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:B970MQT365) STREPTOCOCCUS PNEUMONIAE TYPE 19A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 20A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: Q7FD7W9Y87) (STREPTOCOCCUS PNEUMONIAE TYPE 20A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:Q7FD7W9Y87) STREPTOCOCCUS PNEUMONIAE TYPE 20A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 22F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: U1E9VSB2K2) (STREPTOCOCCUS PNEUMONIAE TYPE 22F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:U1E9VSB2K2) STREPTOCOCCUS PNEUMONIAE TYPE 22F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 23A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: ZPE64MQ45X) (STREPTOCOCCUS PNEUMONIAE TYPE 23A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:ZPE64MQ45X) STREPTOCOCCUS PNEUMONIAE TYPE 23A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 23B CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: R5DF4G8EKE) (STREPTOCOCCUS PNEUMONIAE TYPE 23B CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:R5DF4G8EKE) STREPTOCOCCUS PNEUMONIAE TYPE 23B CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 24F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: A4Z5DLF5NN) (STREPTOCOCCUS PNEUMONIAE TYPE 24F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:A4Z5DLF5NN) STREPTOCOCCUS PNEUMONIAE TYPE 24F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 31 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: TC57VWJ5UQ) (STREPTOCOCCUS PNEUMONIAE TYPE 31 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:TC57VWJ5UQ) STREPTOCOCCUS PNEUMONIAE TYPE 31 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 33F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: 9WP2BC3I04) (STREPTOCOCCUS PNEUMONIAE TYPE 33F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:9WP2BC3I04) STREPTOCOCCUS PNEUMONIAE TYPE 33F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 35B CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: 88A5CHB5FJ) (STREPTOCOCCUS PNEUMONIAE TYPE 35B CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:88A5CHB5FJ) STREPTOCOCCUS PNEUMONIAE TYPE 35B CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL CORYNEBACTERIUM DIPHTHERIAE CRM197 PROTEIN (UNII: 08VC9WC084) (CORYNEBACTERIUM DIPHTHERIAE CRM197 PROTEIN - UNII:08VC9WC084) CORYNEBACTERIUM DIPHTHERIAE CRM197 PROTEIN 65 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) POLYSORBATE 20 (UNII: 7T1F30V5YH) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-4347-01 1 in 1 CARTON 1 NDC:0006-4347-99 0.5 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC:0006-4347-02 10 in 1 CARTON 2 NDC:0006-4347-99 0.5 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125814 06/17/2024 Labeler - Merck Sharp & Dohme LLC (118446553)