Label: M-M-R II- measles, mumps, and rubella virus vaccine live injection, powder, lyophilized, for suspension
- NDC Code(s): 83703-043-00, 83703-043-01
- Packager: Bamboo US BidCo LLC
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated June 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
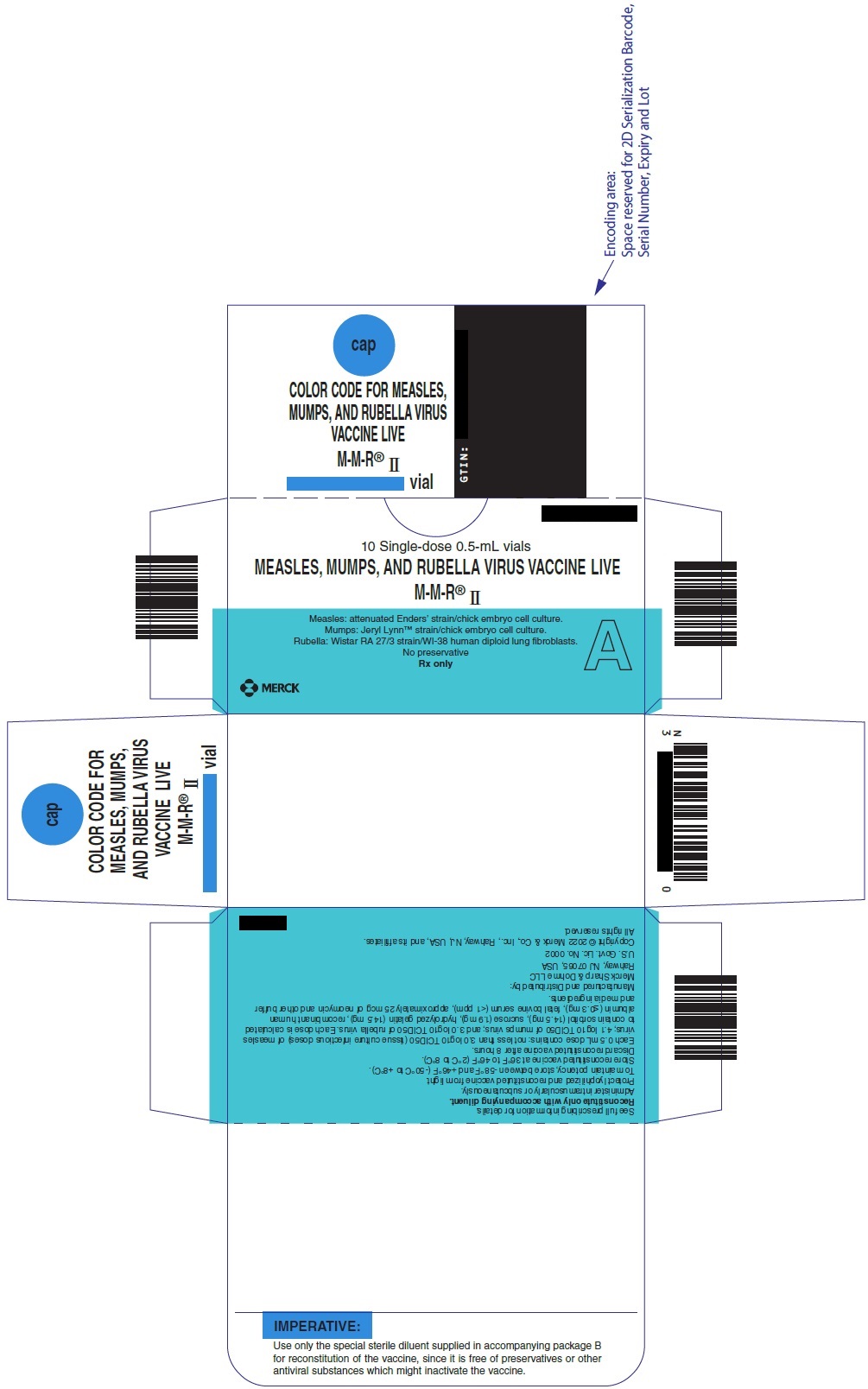
PRINCIPAL DISPLAY PANEL - 0.5 mL Vial Carton
10 Single-dose 0.5-mL vials
MEASLES, MUMPS, AND RUBELLA VIRUS VACCINE LIVE
M-M-R ®IIMeasles: attenuated Enders' strain/chick embryo cell culture.
Mumps: Jeryl Lynn ™strain/chick embryo cell culture.
Rubella: Wistar RA 27/3 strain/WI-38 human diploid lung fibroblasts.
No preservative
Rx onlyA
MERCK
-
INGREDIENTS AND APPEARANCE
M-M-R II
measles, mumps, and rubella virus vaccine live injection, powder, lyophilized, for suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:83703-043 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MEASLES VIRUS STRAIN ENDERS' ATTENUATED EDMONSTON LIVE ANTIGEN (UNII: MFZ8I7277D) (MEASLES VIRUS STRAIN ENDERS' ATTENUATED EDMONSTON LIVE ANTIGEN - UNII:MFZ8I7277D) MEASLES VIRUS STRAIN ENDERS' ATTENUATED EDMONSTON LIVE ANTIGEN 1000 [TCID_50] in 0.5 mL MUMPS VIRUS STRAIN B LEVEL JERYL LYNN LIVE ANTIGEN (UNII: 47QB6MX9KU) (MUMPS VIRUS STRAIN B LEVEL JERYL LYNN LIVE ANTIGEN - UNII:47QB6MX9KU) MUMPS VIRUS STRAIN B LEVEL JERYL LYNN LIVE ANTIGEN 12500 [TCID_50] in 0.5 mL RUBELLA VIRUS STRAIN WISTAR RA 27/3 LIVE ANTIGEN (UNII: 52202H034Z) (RUBELLA VIRUS STRAIN WISTAR RA 27/3 LIVE ANTIGEN - UNII:52202H034Z) RUBELLA VIRUS STRAIN WISTAR RA 27/3 LIVE ANTIGEN 1000 [TCID_50] in 0.5 mL Inactive Ingredients Ingredient Name Strength ALBUMIN BOVINE (UNII: 27432CM55Q) ALBUMIN HUMAN (UNII: ZIF514RVZR) 0.3 mg in 0.5 mL GELATIN, UNSPECIFIED (UNII: 2G86QN327L) 14.5 mg in 0.5 mL NEOMYCIN (UNII: I16QD7X297) 25 ug in 0.5 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE (UNII: SE337SVY37) SORBITOL (UNII: 506T60A25R) 14.5 mg in 0.5 mL SUCROSE (UNII: C151H8M554) 1.9 mg in 0.5 mL Product Characteristics Color yellow (clear yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83703-043-00 10 in 1 CARTON 1 NDC:83703-043-01 0.5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101069 04/21/1971 Labeler - Bamboo US BidCo LLC (119087615)

