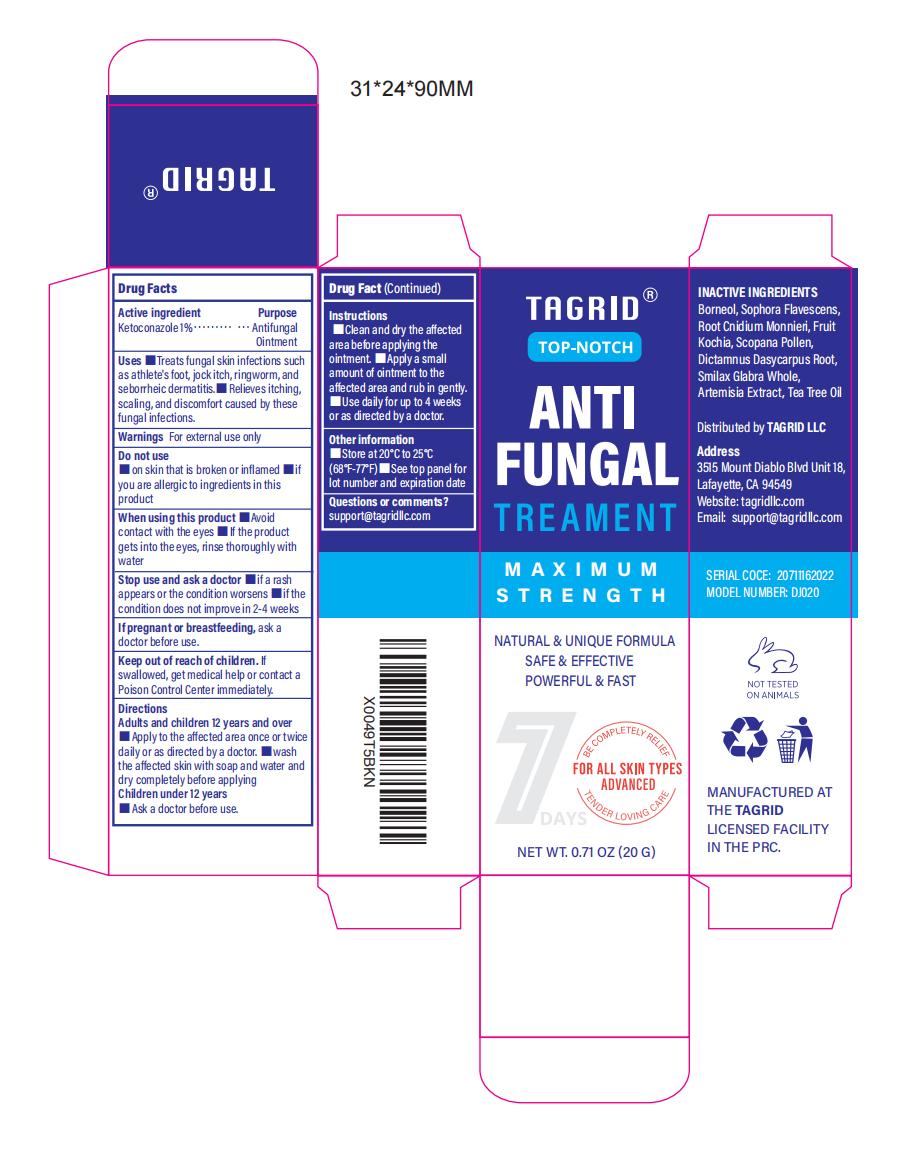
Label: 1% KETOCONAZOLE ANTIFUNGAL TREATMENT- antifungal treatment ointment
- NDC Code(s): 82372-012-01
- Packager: Good Manager Holdings Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated August 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- When Using
- Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
-
Directions
Adults and children 12 years and over
1. Apply to the affected area once or twice daily or as directed by a doctor.
2. Wash the affected skin with soap and water and dry completely before applying.
Children under 12 years old. Ask a doctor before use.Instructions:
1 Clean and dry the affected area before applying the ointment.
2. Apply a small amount of ointment to the affected area and rub in gently.
3. Use daily for up to 4 weeks or as directed by a doctor. - Other information
- Inactive ingredients
- Questions
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
1% KETOCONAZOLE ANTIFUNGAL TREATMENT
antifungal treatment ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82372-012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength KETOCONAZOLE (UNII: R9400W927I) (KETOCONAZOLE - UNII:R9400W927I) KETOCONAZOLE 1 g in 100 g Inactive Ingredients Ingredient Name Strength BORNEOL (UNII: M89NIB437X) WORMWOOD (UNII: F84709P2XV) TEA TREE OIL (UNII: VIF565UC2G) CNIDIUM OFFICINALE ROOT OIL (UNII: 18602BEQ40) BASSIA SCOPARIA FRUIT (UNII: 04W97Z676Y) SMILAX GLABRA WHOLE (UNII: H51N91QNEB) SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) SCOPINE (UNII: Z5LGM3Q28U) DICTAMNUS DASYCARPUS ROOT (UNII: 6153LEN214) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82372-012-01 20 g in 1 TUBE; Type 0: Not a Combination Product 08/19/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 08/19/2024 Labeler - Good Manager Holdings Inc (118382673) Establishment Name Address ID/FEI Business Operations Good Manager Holdings Inc 118382673 manufacture(82372-012)