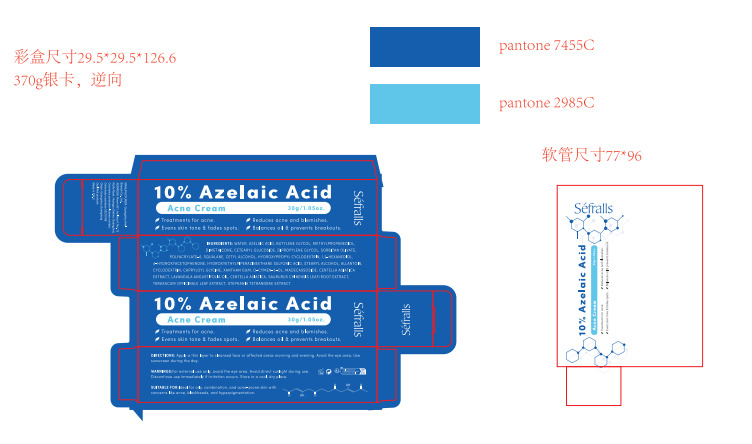
Label: 10% AZELAIC ACID ACNE CREAM cream
- NDC Code(s): 84148-009-01
- Packager: Guangzhou Ariel Biotech Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active Ingredient(s)
AZELAIC ACID 10.00000%
HYDROXYETHYLPIPERAZINE ETHANE SULFONIC ACID 0.50000%
CAPRYLOYL GLYCINE 0.15000%
o-CYMEN-5-OL 0.09000%
MADECASSOSIDE 0.05500%
CENTELLA ASIATICA EXTRACT 0.03000%
LAVANDULA HYBRIDA OIL 0.03000%
ASIATICOSIDE 0.01500%
SAURURUS CHINENSIS LEAF/ROOT EXTRACT 0.01000%
TARAXACUM OFFICINALE (DANDELION) LEAF EXTRACT 0.01000%
STEPHANIA TETRANDRA EXTRACT 0.00160%
- Purpose
- Use
- Warnings
- Do not use
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
-
Inactive ingredients
WATER, AZEL AIC ACID, BUTYLENE GLYCOL, METHYLPROPANEDIOL, DIMETHICONE, CETEARYL GLUCOSIDE, DIPROPYLENE GLYCOL, SORBITAN OLIVATE, POLYACRYLATE-6, SQUAL ANE, CETYL ALCOHOL, HYDROXYPROPYL CYCLODEXTRIN, 1,2-HEXANEDIOL, 4-HYDROXYACETOPHENONE, HYDROXYETHYLPIPERAZINEETHANE SULFONIC ACID, STEARYL ALCOHOL, ALLANTOIN, CYCLODEXTRIN, CAPRYLOYL GLYCINE, XANTHAN GUM, O-CYMEN-5-OL, MADECASSOSIDE, CENTELLA ASIATICA EXTRACT, LAVANDULA ANGUSTIFOLIA OL, CENTELLA ASIATICA, SAURURUS CHINENSIS LEAF/ROOT EXTRACT, TARAXACUM OFFICINALE LEAF EXTRACT, STEPHANIA TETRANDRAE EXTRACT
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
10% AZELAIC ACID ACNE CREAM
10% azelaic acid acne cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84148-009 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASIATICOSIDE (UNII: PKO39VY215) (ASIATICOSIDE - UNII:PKO39VY215) ASIATICOSIDE 0.015 g in 100 g MADECASSOSIDE (UNII: CQ2F5O6YIY) (MADECASSOSIDE - UNII:CQ2F5O6YIY) MADECASSOSIDE 0.055 g in 100 g CENTELLA ASIATICA TRITERPENOIDS (UNII: 4YS74Q4G4J) (CENTELLA ASIATICA TRITERPENOIDS - UNII:4YS74Q4G4J) CENTELLA ASIATICA TRITERPENOIDS 0.03 g in 100 g LAVANDIN OIL (UNII: 9RES347CKG) (LAVANDIN OIL - UNII:9RES347CKG) LAVANDIN OIL 0.03 g in 100 g AZELAIC ACID (UNII: F2VW3D43YT) (AZELAIC ACID - UNII:F2VW3D43YT) AZELAIC ACID 10 g in 100 g O-CYMEN-5-OL (UNII: H41B6Q1I9L) (O-CYMEN-5-OL - UNII:H41B6Q1I9L) O-CYMEN-5-OL 0.09 g in 100 g CAPRYLOYL GLYCINE (UNII: 8TY5YO42NJ) (CAPRYLOYL GLYCINE - UNII:8TY5YO42NJ) CAPRYLOYL GLYCINE 0.15 g in 100 g HYDROXYETHYLPIPERAZINE ETHANE SULFONIC ACID (UNII: RWW266YE9I) (HYDROXYETHYLPIPERAZINE ETHANE SULFONIC ACID - UNII:RWW266YE9I) HYDROXYETHYLPIPERAZINE ETHANE SULFONIC ACID 0.5 g in 100 g TARAXACUM OFFICINALE LEAF (UNII: 0022LFJ74Y) (TARAXACUM OFFICINALE LEAF - UNII:0022LFJ74Y) TARAXACUM OFFICINALE LEAF 0.01 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIPROPYLENE GLYCOL (UNII: E107L85C40) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) CYCLODEXTRINS (UNII: 7E6SK9QDT8) ALLANTOIN (UNII: 344S277G0Z) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) WATER (UNII: 059QF0KO0R) CETYL ALCOHOL (UNII: 936JST6JCN) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) XANTHAN GUM (UNII: TTV12P4NEE) SORBITAN OLIVATE (UNII: MDL271E3GR) SQUALANE (UNII: GW89575KF9) DIMETHICONE (UNII: 92RU3N3Y1O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84148-009-01 30 g in 1 BOTTLE; Type 0: Not a Combination Product 09/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/29/2024 Labeler - Guangzhou Ariel Biotech Co., Ltd. (402818771) Establishment Name Address ID/FEI Business Operations Guangzhou Ariel Biotech Co., Ltd. 402818771 manufacture(84148-009)