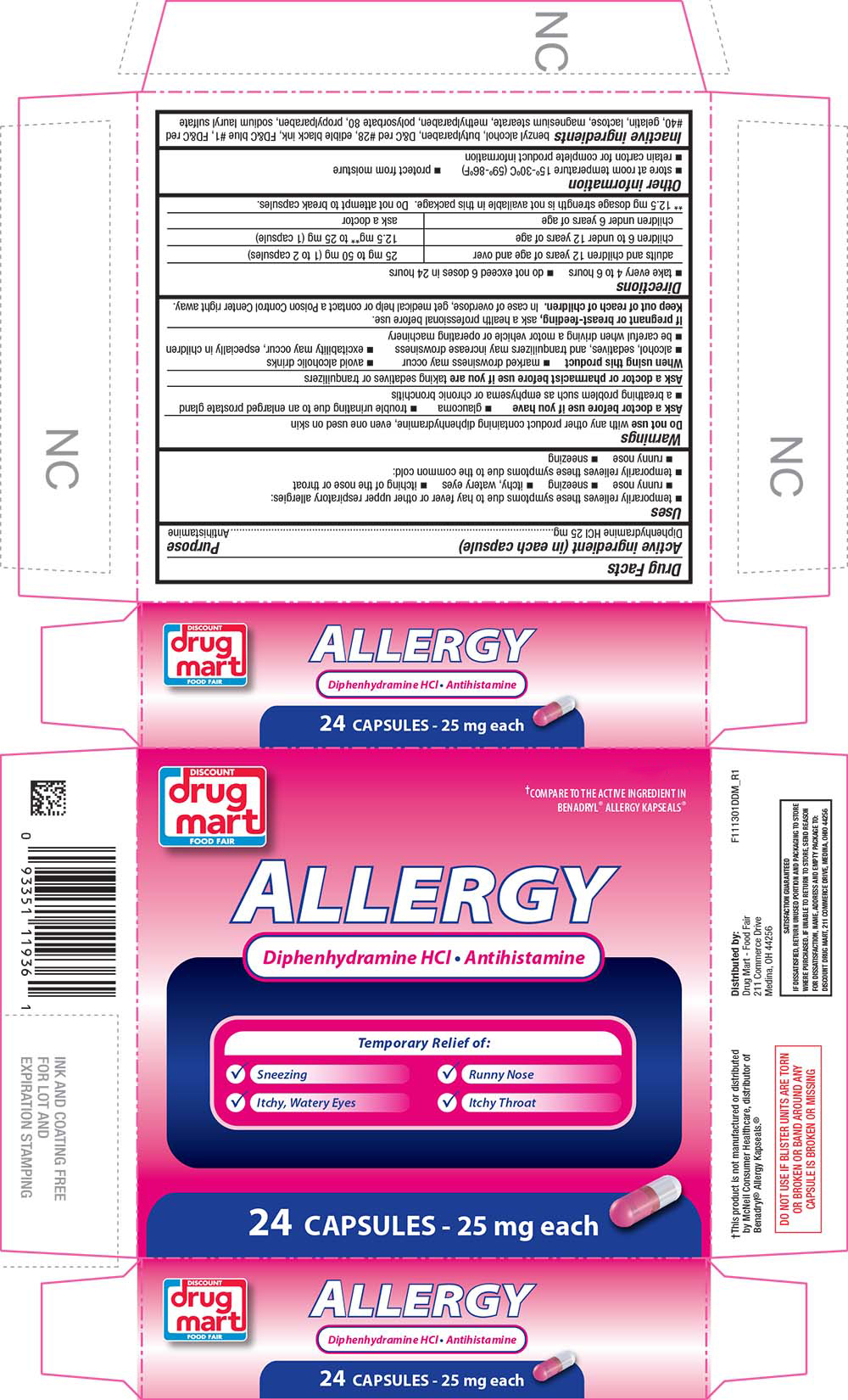
Label: ALLERGY- diphenhydramine hydrochloride capsule
- NDC Code(s): 53943-113-01, 53943-113-03
- Packager: DISCOUNT DRUG MART
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each capsule)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
- Directions
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALLERGY
diphenhydramine hydrochloride capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53943-113 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) BUTYLPARABEN (UNII: 3QPI1U3FV8) D&C RED NO. 28 (UNII: 767IP0Y5NH) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN (UNII: 2G86QN327L) LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) METHYLPARABEN (UNII: A2I8C7HI9T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Product Characteristics Color pink, white Score no score Shape OVAL Size 14mm Flavor Imprint Code A;20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53943-113-01 2 in 1 CARTON 11/30/2007 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:53943-113-03 1 in 1 CARTON 11/30/2007 2 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 11/30/2007 Labeler - DISCOUNT DRUG MART (047741335)