Label: PROVAYBLUE- methylene blue injection
-
NDC Code(s):
0517-0125-01,
0517-0125-05,
0517-0371-01,
0517-0371-05, view more0517-0374-01, 0517-0374-05, 0517-0381-01, 0517-0381-05
- Packager: American Regent, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use (PROVAYBLUE) safely and effectively. See full prescribing information for (PROVAYBLUE).
PROVAYBLUE® (methylene blue) injection, USP for intravenous use
Initial U.S. Approval: 2016WARNING: SEROTONIN SYNDROME WITH CONCOMITANT USE OF SEROTONERGIC DRUGS AND OPIOIDS
See full prescribing information for complete boxed warning.
PROVAYBLUE may cause serious or fatal serotonergic syndrome when used in combination with serotonergic drugs and opioids. Avoid concomitant use of PROVAYBLUE with selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors (MAOIs) and opioids. (5.1, 7.1)
RECENT MAJOR CHANGES
Boxed Warning 11/2023
Indications and Usage (1) 01/2024
Warnings and Precautions (5) 11/2023INDICATIONS AND USAGE
PROVAYBLUE (methylene blue) is an oxidation-reduction agent indicated for the treatment of pediatric and adult patients with acquired methemoglobinemia. (1)
DOSAGE AND ADMINISTRATION
- Administer 1 mg/kg intravenously over 5-30 minutes. (2.1)
- If methemoglobin level remains above 30% or if clinical symptoms persist, give a repeat dose of up to 1 mg/kg one hour after the first dose. (2.1)
- Administer a single dose of 1 mg/kg in patients with moderate or severe renal impairment. (2.2)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
PROVAYBLUE is contraindicated in the following conditions (4):
- Severe hypersensitivity to methylene blue
- Patients with glucose-6-phosphate dehydrogenase deficiency (G6PD) due to the risk of hemolytic anemia
WARNINGS AND PRECAUTIONS
- Hypersensitivity: If severe or life threatening allergic reaction occurs, discontinue PROVAYBLUE, treat the allergic reaction, and monitor until signs and symptoms resolve (5.2)
- Lack of Effectiveness: Consider alternative treatments if there is no resolution of methemoglobinemia after 2 doses (2.1, 5.3)
- Hemolytic Anemia: Discontinue PROVAYBLUE and transfuse (5.4)
- Interference with In-Vivo Monitoring Devices: Use methods other than pulse oximetry to assess oxygen saturation (5.5)
- Effects on Ability to Drive and Operate Machinery: Advise patients to refrain from these activities until neurologic and visual symptoms have resolved (5.6)
ADVERSE REACTIONS
The most commonly reported adverse reactions (>2%) included headache, hypokalemia, diarrhea, hypomagnesemia, myoclonus, nausea, and seizure-like phenomena. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact American Regent at 1-800-734-9236, FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SEROTONIN SYNDROME WITH CONCOMITANT USE OF SEROTONERGIC DRUGS AND OPIOIDS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage and Administration
2.2 Recommended Dosage for Renal Impairment
2.3 Preparation
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serotonin Syndrome with Concomitant Use of Serotonergic Drugs and Opioids
5.2 Hypersensitivity
5.3 Lack of Effectiveness
5.4 Hemolytic Anemia
5.5 Interference with In Vivo Monitoring Devices
5.6 Effects on Ability to Drive and Operate Machinery
5.7 Interference with Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Treatment of Acquired Methemoglobinemia
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: SEROTONIN SYNDROME WITH CONCOMITANT USE OF SEROTONERGIC DRUGS AND OPIOIDS
PROVAYBLUE may cause serious or fatal serotonergic syndrome when used in combination with serotonergic drugs and opioids. Avoid concomitant use of PROVAYBLUE with selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors (MAOIs) and opioids. [see Warnings and Precautions (5.1) and Drug Interactions (7.1)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage and Administration
- Ensure patent venous access prior to administration of PROVAYBLUE. Do not administer PROVAYBLUE subcutaneously.
- Administer PROVAYBLUE 1 mg/kg intravenously over 5-30 minutes.
- If the methemoglobin level remains greater than 30% or if clinical signs and symptoms persist, a repeat dose of PROVAYBLUE 1 mg/kg may be given one hour after the first dose.
- If methemoglobinemia does not resolve after 2 doses of PROVAYBLUE, consider initiating alternative interventions for treatment of methemoglobinemia.
2.2 Recommended Dosage for Renal Impairment
- The recommended dosage of PROVAYBLUE in patients with moderate or severe renal impairment (eGFR 15-59 mL/min/1.73 m2) is a single dose of 1 mg/kg.
- If the methemoglobin level remains greater than 30% or if the clinical symptoms persist 1 hour after dosing, consider initiating alternative interventions for the treatment of methemoglobinemia.
2.3 Preparation
PROVAYBLUE is hypotonic and may be diluted before use in a solution of 50 mL 5% Dextrose Injection in order to avoid local pain, particularly in the pediatric population. Use the diluted solution immediately after preparation.
Avoid diluting with sodium chloride solutions, because it has been demonstrated that chloride reduces the solubility of methylene blue.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Discard unused portion.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Serotonin Syndrome with Concomitant Use of Serotonergic Drugs and Opioids
The development of serotonin syndrome has been reported with the use of methylene blue class products. Most reports have been associated with concomitant use of serotonergic drugs (e.g., selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors (MAOIs). Opioids and dextromethorphan may increase the risk of developing serotonin syndrome. Some of the reported cases were fatal. Symptoms associated with serotonin syndrome may include the following combination of signs and symptoms: mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, and hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, and incoordination), seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Avoid concomitant use of PROVAYBLUE with serotonergic drugs and opioids.
Patients treated with PROVAYBLUE should be monitored for the emergence of serotonin syndrome. If symptoms of serotonin syndrome occur, discontinue use of PROVAYBLUE, and initiate supportive treatment. Inform patients of the increased risk of serotonin syndrome and advise them to not to take serotonergic drugs within 72 hours after the last dose of PROVAYBLUE [see Drug Interactions (7), Patient Counseling Information (17)].
5.2 Hypersensitivity
Anaphylactic reactions to methylene blue class products have been reported. Patients treated with PROVAYBLUE should be monitored for anaphylaxis. If anaphylaxis or other severe hypersensitivity reactions (e.g., angioedema, urticaria, bronchospasm) should occur, discontinue use of PROVAYBLUE and initiate supportive treatment. PROVAYBLUE is contraindicated in patients who have experienced anaphylaxis or other severe hypersensitivity reactions to a methylene blue class product in the past.
5.3 Lack of Effectiveness
Methemoglobinemia may not resolve or may rebound after response to treatment with PROVAYBLUE in patients with methemoglobinemia due to aryl amines such as aniline or sulfa drugs such as dapsone. Monitor response to therapy with PROVAYBLUE through resolution of methemoglobinemia. If methemoglobinemia does not respond to 2 doses of PROVAYBLUE or if methemoglobinemia rebounds after a response, consider additional treatment options [see Dosage and Administration (2.2)].
Patients with glucose-6-phosphate dehydrogenase deficiency may not reduce PROVAYBLUE to its active form in vivo. PROVAYBLUE may not be effective in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.
5.4 Hemolytic Anemia
Hemolysis can occur during treatment of methemoglobinemia with PROVAYBLUE. Laboratory testing may show Heinz bodies, elevated indirect bilirubin and low haptoglobin, but the Coombs test is negative. The onset of anemia may be delayed 1 or more days after treatment with PROVAYBLUE. The anemia may require red blood cell transfusions [see Adverse Reactions (6.1)]. Use the lowest effective number of doses of PROVAYBLUE to treat methemoglobinemia. Discontinue PROVAYBLUE and consider alternative treatments of methemoglobinemia if severe hemolysis occurs.
Treatment of patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency with PROVAYBLUE may result in severe hemolysis and severe anemia. PROVAYBLUE is contraindicated for use in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency [see Contraindications (4)].
5.5 Interference with In Vivo Monitoring Devices
- Inaccurate Pulse Oximeter Readings
The presence of methylene blue in the blood may result in an underestimation of the oxygen saturation reading by pulse oximetry. If a measure of oxygen saturation is required during or shortly after infusion of PROVAYBLUE, it is advisable to obtain an arterial blood sample for testing by an alternative method.
- Bispectral index monitor
A fall in the Bispectral Index (BIS) has been reported following administration of methylene blue class products. If PROVAYBLUE is administered during surgery, alternative methods for assessing the depth of anesthesia should be employed.
5.6 Effects on Ability to Drive and Operate Machinery
Treatment with PROVAYBLUE may cause confusion, dizziness and disturbances in vision [see Adverse Reactions (6)]. Advise patients to refrain from driving or engaging in hazardous occupations or activities such as operating heavy or potentially dangerous machinery until such adverse reactions to PROVAYBLUE have resolved.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Serotonin Syndrome with Concomitant Use of Serotonergic Drugs [see Warnings and Precautions (5.1)]
- Anaphylaxis [see Warnings and Precautions (5.2)]
- Lack of Effectiveness [see Warnings and Precautions (5.3)]
- Hemolytic Anemia [see Warnings and Precautions (5.4)]
- Interference with In-Vivo Monitoring Devices [see Warnings and Precautions (5.5)]
- Effects on Ability to Drive and Operate Machinery [see Warnings and Precautions (5.6)]
- Interference with Laboratory Tests [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of PROVAYBLUE in adults with acquired methemoglobinemia was assessed in 31 patients who received at least 1 dose of PROVAYBLUE [see Clinical Studies (14)]. Most doses administered were 1 mg/kg (82.9%), but doses from 0.78 mg/kg to 2 mg/kg were administered. All patients received at least one dose of PROVAYBLUE; two received two doses, and one received three doses. Serious adverse reactions occurred in 3.2% of patients who received PROVAYBLUE. A serious adverse reaction of seizure-like phenomenon was reported in one patient. Adverse reactions (≥2%) included headache, hypokalemia, diarrhea, hypomagnesemia, myoclonus, nausea, and seizure-like phenomena.
The safety of PROVAYBLUE in pediatric patients with acquired methemoglobinemia was assessed in two retrospective case series that included two pediatric patients treated with PROVAYBLUE and 12 treated with another methylene blue product. The case series included patients in the following age groups: 3 neonates (<1 month), 4 infants (1 month to <2 years), 4 children (2 years to <12 years), and 3 adolescents (12 years to <17 years). The safety profile in pediatric patients was similar to that in adult patients.
Other adverse reactions reported to occur following the administration of methylene blue class products include the following:
Blood and lymphatic system disorders: hemolytic anemia, hemolysis, hyperbilirubinemia
Cardiac disorders: palpitations, tachycardia
Eye disorders: eye pruritus, ocular hyperemia, vision blurred
Gastrointestinal disorders: abdominal pain lower, dry mouth, flatulence, glossodynia, tongue eruption
General disorders and administration site conditions: death, infusion site extravasation, infusion site induration, infusion site pruritus, infusion site swelling, infusion site urticaria, peripheral swelling, thirst
Investigations: elevated liver enzymes
Musculoskeletal and connective tissue disorders: myalgia
Renal and urinary disorders: dysuria
Respiratory, thoracic and mediastinal disorders: nasal congestion, oropharyngeal pain, rhinorrhea, sneezing
Skin and subcutaneous tissue disorders: necrotic ulcer, papule, phototoxicity
Vascular disorders: hypertension
-
7 DRUG INTERACTIONS
Clinically significant drug interactions with PROVAYBLUE are described below:
The concomitant use of PROVAYBLUE with other drugs that affect the serotonergic neurotransmitter system has resulted in serotonin syndrome. Although the mechanism is not clearly understood, literature reports suggest PROVAYBLUE is a potent reversible inhibitor of monoamine oxidase. Avoid concomitant use of PROVAYBLUE with medicinal products that enhance serotonergic transmission including antidepressants like SSRIs (selective serotonin reuptake inhibitors), SNRIs (serotonin and norepinephrine reuptake inhibitors), MAOIs (monoamine oxidase inhibitors), bupropion, buspirone, clomipramine, mirtazapine, linezolid, opioids, and dextromethorphan because of the potential for serious CNS reactions, including potentially fatal serotonin syndrome. If the intravenous use of PROVAYBLUE cannot be avoided in patients treated with serotonergic medicinal products, choose the lowest possible dose and observe the patient closely for CNS effects for up to 4 hours after administration [see Warning and Precautions (5.1) and Clinical Pharmacology (12.3)]. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
PROVAYBLUE may cause fetal harm when administered to a pregnant woman. Intra-amniotic injection of pregnant women with a methylene blue class product during the second trimester was associated with neonatal intestinal atresia and fetal death. Methylene blue produced adverse developmental outcomes in rats and rabbits when administered orally during organogenesis at doses at least 32 and 16 times, respectively, the clinical dose of 1 mg/kg (see Data). Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies are 2-4% and 15-20%, respectively.
Clinical Considerations
Fetal/neonatal adverse reactions
Intra-amniotic injection of a methylene blue class product hours to days prior to birth can result hyperbilirubinemia, hemolytic anemia, skin staining, methemoglobinemia, respiratory distress and photosensitivity in the newborn. Following administration of PROVAYBLUE to a pregnant woman at term, observe the newborn for these adverse reactions and institute supportive care.
Data
Animal Data
Methylene blue was administered orally to pregnant rats at doses of 50 to 350 mg/kg/day, during the period of organogenesis. Maternal and embryofetal toxicities were observed at all doses of methylene blue and were most evident at the 200 and 350 mg/kg/day doses. Maternal toxicity consisted of increased spleen weight. Embryo-fetal toxicities included reduced fetal weight, post-implantation loss, edema, and malformations including enlarged lateral ventricles. The dose of 200 mg/kg (1200 mg/m2) in rats is approximately 32 times a clinical dose of 1 mg/kg based on body surface area.
Methylene blue was administered orally to pregnant rabbits at doses of 50, 100, or 150 mg/kg/day, during the period of organogenesis. Maternal death was observed at the methylene blue dose of 100 mg/kg. Embryofetal toxicities included spontaneous abortion at all dose levels and a malformation (umbilical hernia) at the 100 and 150 mg/kg/day doses. The dose of 50 mg/kg (600 mg/m2) in rabbits is approximately 16 times a clinical dose of 1 mg/kg based on body surface area.
8.2 Lactation
Risk Summary
There is no information regarding the presence of methylene blue in human milk, the effects on the breastfed infant, or the effects on milk production. Because of the potential for serious adverse reactions including genotoxicity, discontinue breast-feeding during and for up to 8 days after treatment with PROVAYBLUE [see Clinical Pharmacology (12.3)].
8.4 Pediatric Use
The safety and effectiveness of PROVAYBLUE for the treatment of acquired methemoglobinemia have been established in pediatric patients. Use of PROVAYBLUE is supported by two retrospective case series that included 2 pediatric patients treated with PROVAYBLUE and 12 treated with another methylene blue class product. The case series included pediatric patients in the following age groups: 3 neonates (less than 1 month), 4 infants (1 month up to less than 2 years), 4 children (2 years up to less than 12 years), and 3 adolescents (12 years to less than 17 years). The efficacy outcomes were consistent across pediatric and adult patients in both case series [see Clinical Studies (14)].
8.5 Geriatric Use
Clinical studies of PROVAYBLUE did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. PROVAYBLUE is known to be substantially excreted by the kidney, so the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, treatment of methemoglobinemia in these patients should use the lowest number of doses needed to achieve a response [see Dosage and Administration (2)].
8.6 Renal Impairment
Methylene blue concentrations increased in subjects with renal impairment (eGFR 15 to 89 mL/min/1.73m2) significantly [see Clinical Pharmacology (12.3)]. Adjust PROVAYBLUE dosage in patients with moderate or severe renal impairment (eGFR 15 to 59 mL/min/1.73 m2) [see Dosage and Administration (2.2)]. No dose adjustment is recommended in patients with mild renal impairment (eGFR 60 – 89 mL/min/1.73 m2).
-
10 OVERDOSAGE
Hypotension, wheezing and reduced oxygenation have been reported in patients who received methylene blue class products in single doses of 3 mg/kg or more.
Administration of large intravenous doses (cumulative dose ≥ 7 mg/kg ) of a methylene blue class product caused nausea, vomiting, precordial pain, dyspnea, tachypnea, chest tightness, tachycardia, apprehension, tremor, mydriasis, blue staining of the urine, the skin and mucous membranes, abdominal pain, dizziness, paresthesia, headache, confusion, mild methemoglobinemia (up to 7%) and electrocardiogram changes (T-wave flattening or inversion). These effects lasted 2-12 hours following administration.
A severe overdosage (single dose of 20 mg/kg or more) of a methylene blue class product caused severe intravascular hemolysis, hyperbilirubinemia and death.
In case of overdose of PROVAYBLUE, maintain the patient under observation until signs and symptoms have resolved, monitor for cardiopulmonary, hematologic and neurologic toxicities, and institute supportive measures as necessary.
-
11 DESCRIPTION
Methylene blue is an oxidation-reduction agent.
Its chemical name is 3,7-bis(dimethylamino)phenothiazin-5-ium, chloride hydrate. The molecular formula of methylene blue is C16H18ClN3S.xH2O and its molecular weight of 319.86 g/mol for the anhydrous form. The structural formula of methylene blue is:
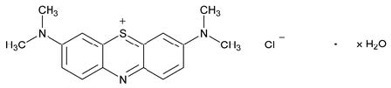
PROVAYBLUE (methylene blue) injection USP is a sterile solution intended for intravenous administration. Each mL of solution contains 5 mg methylene blue and water for injection. PROVAYBLUE (methylene blue) injection, USP: is a clear dark blue solution with a pH value between 3.0 and 4.5. The osmolality is between 10 and 15 mOsm/kg. PROVAYBLUE (methylene blue) injection strength is expressed in terms of trihydrate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Methylene blue is a water soluble thiazine dye that promotes a non-enyzmatic redox conversion of metHb to hemoglobin. In situ, methylene blue is first converted to leucomethylene blue (LMB) via NADPH reductase. It is the LMB molecule which then reduces the ferric iron of metHb to the ferrous state of normal hemoglobin.
12.2 Pharmacodynamics
Low concentrations of methylene blue speeds up the in vivo conversion of methemoglobin to hemoglobin. Methylene blue has been observed to stain tissues selectively. The exposure-response or –safety relationship for methylene is unknown.
12.3 Pharmacokinetics
The mean (CV%) Cmax and AUC of methylene blue 2,917 ng/mL (39%) and 13977 ng.hr/mL (21%) following a 2 mg/kg dose administered as a 5-minute intravenous infusion.
Distribution
The mean± standard deviation steady state volume of distribution of a 2 mg/kg dose of PROVAYBLUE was 255 L ± 58. The mean plasma protein binding of methylene blue is approximately 94% in vitro. Methylene blue exhibits concentration-dependent partitioning into blood cells in vitro. The blood-to-plasma ratio was 5.1±2.8 at 5 minutes from the start of a 2 mg/kg dose administered as a 5-minute intravenous infusion and reached a plateau of 0.6 at 4 hours in a clinical study. Methylene Blue is a substrate for the P-glycoprotein (P-gp, ABCB1) transporter, but not for BCRP or OCT2 in vitro.
Metabolism
Methylene blue is metabolized by CYPs 1A2, 2C19 and 2D6 in vitro; however, the predominant in vitro pathway appears to be UGT-mediated conjugation by multiple UGT enzymes, including UGT1A4 and UGT1A9.
Azure B, which is a minor impurity in methylene blue, is also formed in humans as a metabolite of methylene blue, with an overall drug/metabolite AUC ratio of greater than 6:1. Azure B has 8-fold lower potency than methylene blue.
Specific Populations
Renal Impairment
After a single 1 mg/kg dose of PROVAYBLUE, AUC0-96h increased by 52%, 116%, and 192% in subjects with mild (estimated glomerular filtration rate (eGFR) 60 – 89 mL/min/1.73 m2), moderate (eGFR 30-59 mL/min/1.73m2), and severe (eGFR 15-29 mL/min/1.732m2) renal impairment, respectively. Cmax increased by 42%, 34%, and 15% in subjects with mild, moderate, and severe renal impairment respectively [see Dosage and Administration (2.2) and Use in Specific Populations (8.6)]. The half-life was unchanged in patients with mild to moderate renal impairment.
The AUC0-96h of Azure B after a single 1 mg/kg dose increased by 29%, 94%, and 339% in subjects with mild (estimated glomerular filtration rate (eGFR) 60 – 89 mL/min/1.73 m2), moderate (eGFR 30-59 mL/min/1.73m2), and severe (eGFR 15-29 mL/min/1.732m2) renal impairment, respectively. Cmax increased by 23%, 13%, and 65% in subjects with mild, moderate, and severe renal impairment, respectively [see Dosage and Administration (2.2) and Use in Specific Populations (8.6)]Drug Interactions Studies
Clinical Studies:
The coadministration of 2 mg/kg dose of PROVAYBLUE with midazolam (a CYP3A4 substrate), caffeine (a CYP1A2 substrate), warfarin (a CYP2C9 substrate), and dextromethorphan (a CYP2D6 substrate) in a cocktail study did not affect the exposure of these substrates compared to their exposure without PROVAYBLUE administration.In Vitro Studies:
Cytochrome P450 (CYP450) Enzymes:
Methylene blue inhibits CYP isozymes 1A2, 2B6, 2C8, 2C9, 2C19, 2D6 and 3A4/5. Possible time-dependent inhibition of CYP2C9, CYP2D6 and CYP3A4/5 (testosterone as substrate) was also observed. Methylene blue induces CYP1A2 but does not induce CYP2B6 or CYP3A4.
UDP-Glucuronosyltransferase (UGT):
Methylene blue inhibits UGT1A9 and UGT1A4, but did not significantly inhibit UGTs 1A1, 1A3, 1A6, 2B7 or 2B15.Transporter:
Methylene blue is both a substrate for and an inhibitor of P-gp but is not a substrate for BCRP or OCT2 in vitro. Methylene blue is not a significant inhibitor of BCRP, OAT1, OAT3, OAT1B1 or OAT1B3. Methylene blue inhibits OCT2, MATE1 and MATE2-K.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a two-year carcinogenicity study, rats were administered oral doses of methylene blue at 5, 25, or 50 mg/kg. Methylene blue caused pancreatic islet adenomas or carcinomas (combined) in male rats. In a two-year carcinogenicity study, mice were administered oral doses of methylene blue at 2.5, 12.5, or 25 mg/kg. There were no drug-related neoplastic findings in mice.
Methylene blue was genotoxic in gene mutation assays in bacteria (Ames test), and in an in vitro sister chromatid exchange test and an in vitro chromosomal aberration test in Chinese hamster ovary (CHO) cells. Methylene blue was negative for micronucleus induction in bone marrow or peripheral blood collected from mice treated with methylene blue.
Fertility studies with methylene blue have not been conducted. In vitro, methylene blue reduced motility of human sperm in a concentration dependent manner.
-
14 CLINICAL STUDIES
14.1 Treatment of Acquired Methemoglobinemia
The efficacy of PROVAYBLUE in the treatment of patients with methemoglobinemia was evaluated in 31 adult patients with acquired methemoglobinemia across two studies: NCT03395223, a prospective, interventional, open-label, single-arm study, and NCT03542760, a prospective, multicenter, observational registry. Of the 31 subjects enrolled 90% were white, 10% were black, 58% were female, and 42% were male. Hispanic or Latino was 9.7%; non-Hispanic or Latino was 67.7%, and ethnicity data were missing for 22.6%. The mean age was 45.6 years, and the ages ranged from 19 to 72 years. Each individual received at least 1 intravenous dose of PROVAYBLUE; two received 2 doses and one received 3 doses. Most doses administered were 1 mg/kg (82.9%), but doses from 0.78 mg/kg to 2 mg/kg were administered. The recommended PROVAYBLUE dose is 1 mg/kg; lower or greater doses are not recommended. The maximum recommended number of doses is two [see Dosage and Administration (2.1)].
In total, 29 of the 31 (93.5%) subjects had post treatment methemoglobin (metHb) assessment; 28 of the 29 subjects had baseline metHb with a mean concentration of 18.4% and a range of 4.1% to 74.4%. Twenty-six of the 28 (92.9%) subjects who had baseline metHb had at least a 50% reduction in metHB from baseline in their first assessment post baseline. This first post dosing assessment occurred from 0.2 to 27.3 hours from the end of first PROVAYBLUE infusion with a median time of 2.7 hours. There were 12 subjects that had baseline metHb and had metHb assessed within 2 hours of the end of the first PROVAYBLUE treatment; 9 of the 12 (75%; 95% CI (42.8%,93.3%)) had at least a 50% reduction in metHb at 1 hour postdosing.
Available vital sign data including blood pressure, heart rate and respiratory rate were reviewed at baseline and compared to data collected within 2 hours post PROVAYBLUE infusion. Prior to treatment with PROVAYBLUE, 16 of the 23 (70%) of patients had a respiratory rate exceeding the upper limit of normal (≥ 20 bpm). Of these, 10 of the 16 (63%) experienced a normalization of respiratory rate within 2 hours post ProvayBlue infusion. There was minimal impact on other vital signs.
At baseline, the most common prespecified signs and symptoms of methemoglobinemia (reported by ≥2 subjects [6.5%] overall) were cyanosis (32.3%), dyspnea (25.8%), fatigue (25.8%), depressed CNS (9.7%), headache (6.5%), weakness (6.5%), and dizziness (6.5%). Following treatment with PROVAYBLUE, signs and symptoms of methemoglobinemia improved.
The efficacy of PROVAYBLUE in the treatment of methemoglobinemia in pediatric patients was assessed in 14 patients in two retrospective case series (2 patients received PROVAYBLUE and 12 who received another methylene blue product). The ages ranged from 6 days to 16 years. The efficacy outcomes were consistent across the pediatric and adult populations.
-
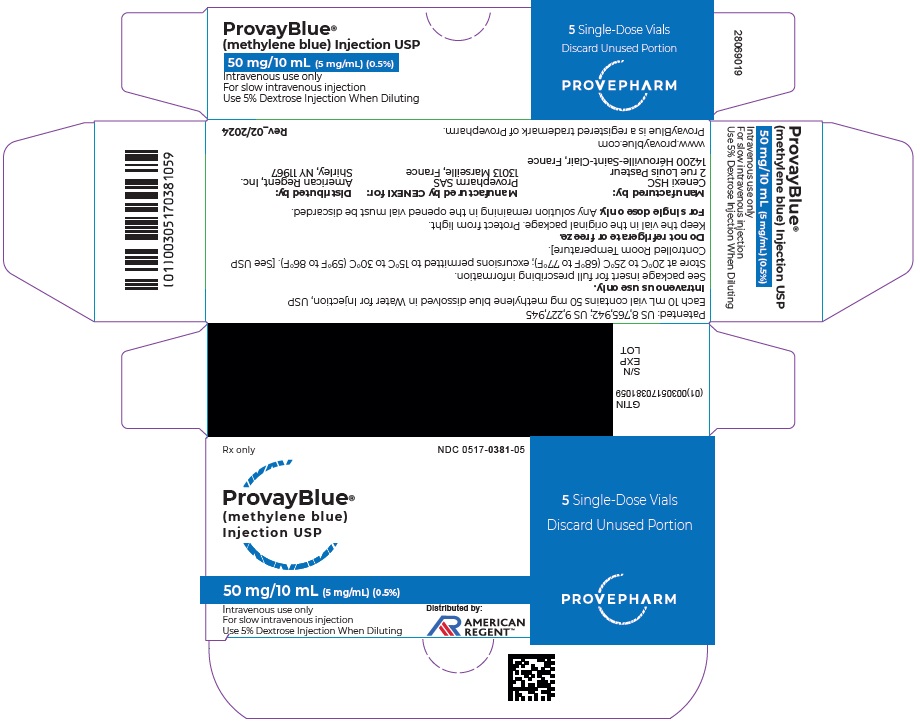
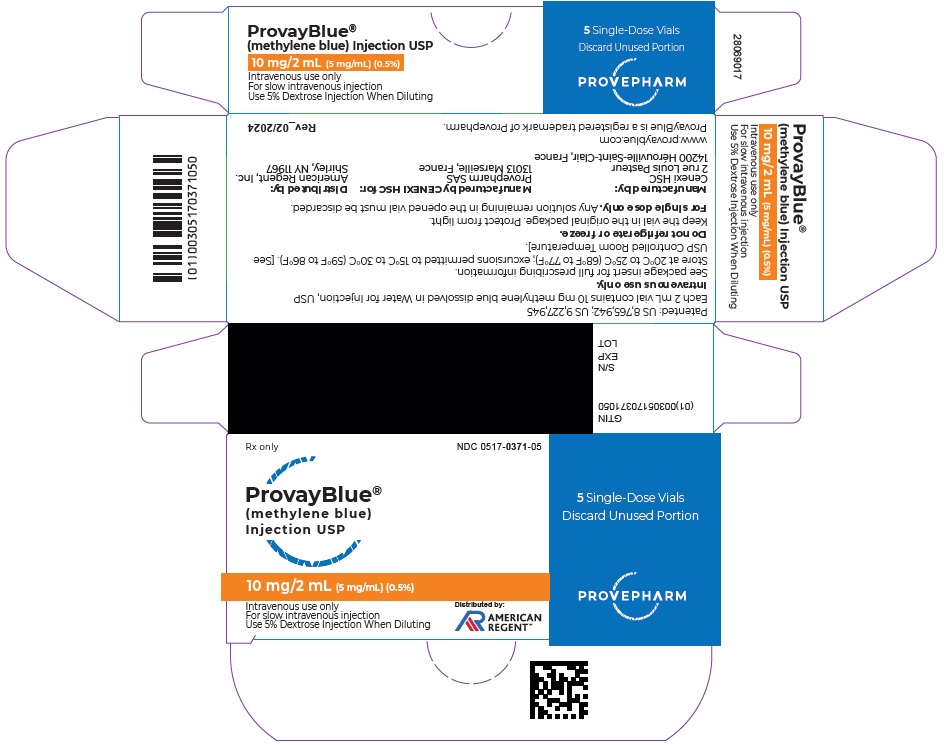
16 HOW SUPPLIED/STORAGE AND HANDLING
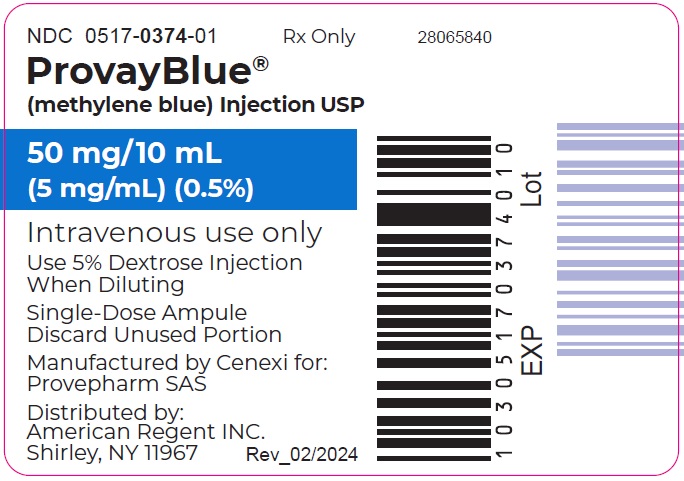
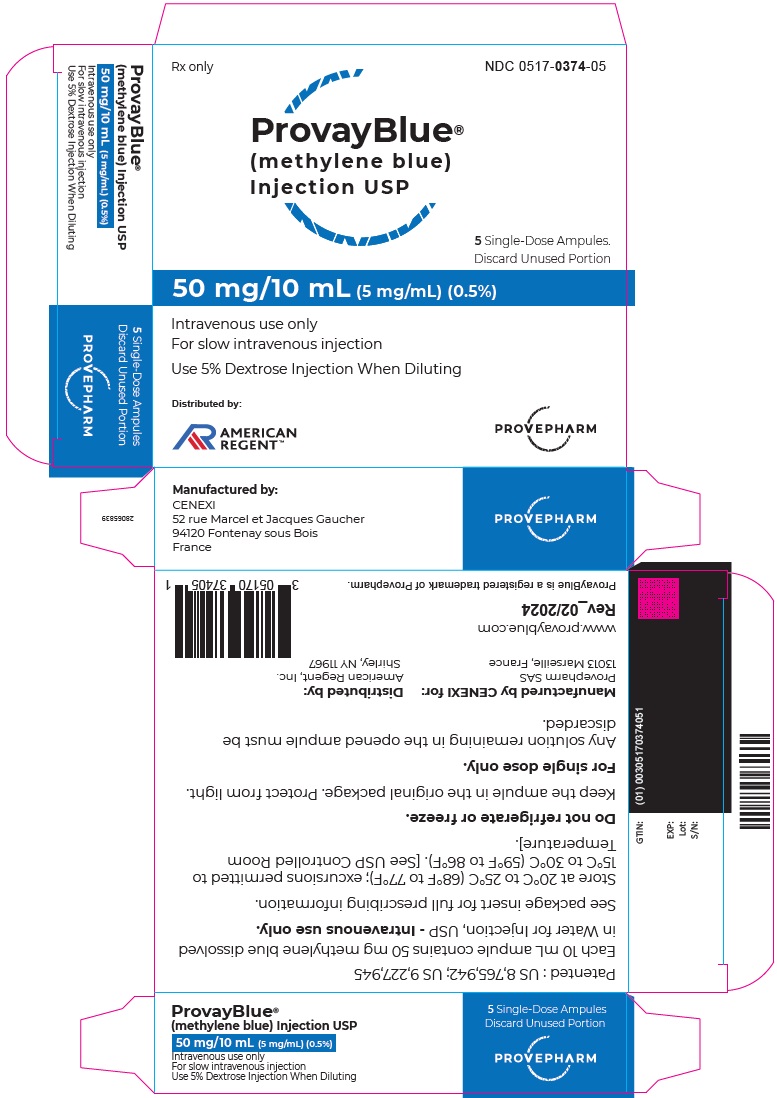
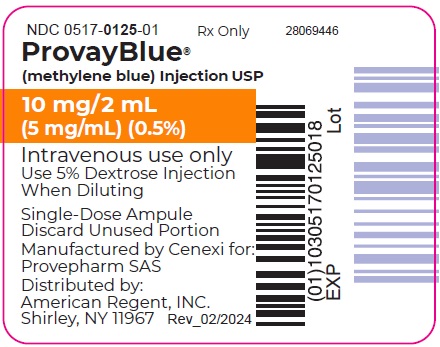
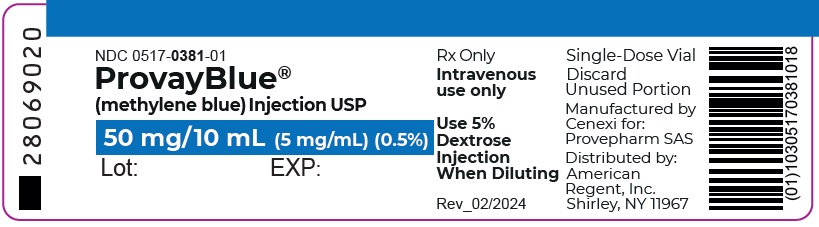
PROVAYBLUE (methylene blue) injection, USP: is supplied in 10 mL and 2 mL single-dose ampules or single-dose vials. Each 10 mL ampule and vial contains 50 mg of methylene blue as a clear dark blue solution. Each 2 mL ampule and vial contains 10 mg of methylene blue as a clear dark blue solution. A box contains five ampules or vials.
Box of 5 ampules of 50 mg/10 mL: NDC 0517-0374-05
Box of 5 ampules of 10 mg/2 mL: NDC 0517-0125-05
Box of 5 vials of 50 mg/10 mL: NDC 0517-0381-05
Box of 5 vials of 10 mg/2 mL: NDC 0517-0371-05
Storage:
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). [See USP Controlled Room Temperature]
Any unused product or waste material should be disposed of in accordance with local practice.
Do not refrigerate or freeze.
Keep the ampule or the vial in the original package to protect from light. -
17 PATIENT COUNSELING INFORMATION
Serotonin Syndrome
Advise patients of the possibility of serotonin syndrome, especially with concomitant use of serotonergic agents such as medications to treat depression and migraines. Advise patients to seek immediate medical attention if the following symptoms occur after treatment with PROVAYBLUE: changes in mental status, autonomic instability, or neuromuscular symptoms with or without gastrointestinal symptoms [see Warnings and Precautions (5.1)].
Pregnancy
Advise pregnant women of the potential risk to the fetus with the use of PROVAYBLUE during pregnancy [see Use in Specific populations (8.1)].
Breastfeeding
Advise patients to discontinue breast-feeding for up to 8 days after treatment with PROVAYBLUE [see Use in Specific populations (8.2)].
Driving and Using Machines
Advise patients to avoid driving and use of machines during treatment with PROVAYBLUE. Driving can be affected as a result of a confusional state, dizziness and possible eye disturbances [see Warnings and Precautions (5.6)].
Phototoxicity
Advise patients to take protective measures against exposure to light, because phototoxicity may occur after administration of methylene blue [see Adverse Reactions (6.1)].
Skin and Body Fluid Blue Discoloration
Advise patients that PROVAYBLUE may cause a blue discoloration of the skin and body fluids [see Adverse Reactions (6.1)].
Manufactured for:
PROVEPHARM SAS
22 rue Marc Donadille
13013 Marseille, FranceAmpules manufactured by:
CENEXI
52 rue Marcel et Jacques Gaucher
94120 Fontenay sous Bois, FranceVials manufactured by: CENEXI HSC
2 rue Louis Pasteur
14200 Hérouville-Saint-Clair, FranceDistributed by:
American Regent, Inc.
Shirley, NY 11967
Questions? : 1-800-734-9236 - Principal Display Panel - 50 mg/10 mL (5 mg/mL) Ampule Label
- Principal Display Panel - 50 mg/10 mL (5 mg/mL) Carton Label
- Principal Display Panel - 10 mg/2 mL (5 mg/mL) Ampule Label
- Principal Display Panel - 10 mg/2 mL (5 mg/mL) Carton Label
- Principal Display Panel - 50 mg/10 mL (5 mg/mL) Single-Dose Vial Label
- Principal Display Panel - 50 mg/10 mL (5 mg/mL) Single-Dose Vial Carton Label
- Principal Display Panel - 10 mg/2 mL (5 mg/mL) Single-Dose Vial Label
- Principal Display Panel - 10 mg/2 mL (5 mg/mL) Single-Dose Vial Carton Label
-
INGREDIENTS AND APPEARANCE
PROVAYBLUE
methylene blue injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0517-0374 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYLENE BLUE (UNII: T42P99266K) (METHYLENE BLUE CATION - UNII:ZMZ79891ZH) METHYLENE BLUE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0517-0374-05 5 in 1 CARTON 04/08/2016 1 NDC:0517-0374-01 10 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204630 04/08/2016 PROVAYBLUE
methylene blue injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0517-0381 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYLENE BLUE (UNII: T42P99266K) (METHYLENE BLUE CATION - UNII:ZMZ79891ZH) METHYLENE BLUE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0517-0381-05 5 in 1 CARTON 12/31/2021 1 NDC:0517-0381-01 10 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204630 04/08/2016 PROVAYBLUE
methylene blue injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0517-0125 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYLENE BLUE (UNII: T42P99266K) (METHYLENE BLUE CATION - UNII:ZMZ79891ZH) METHYLENE BLUE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0517-0125-05 5 in 1 CARTON 04/08/2020 1 NDC:0517-0125-01 2 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204630 04/08/2020 PROVAYBLUE
methylene blue injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0517-0371 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYLENE BLUE (UNII: T42P99266K) (METHYLENE BLUE CATION - UNII:ZMZ79891ZH) METHYLENE BLUE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0517-0371-05 5 in 1 CARTON 12/31/2021 1 NDC:0517-0371-01 2 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204630 04/08/2020 Labeler - American Regent, Inc. (002033710) Registrant - Provepharm SAS (296789410) Establishment Name Address ID/FEI Business Operations Cenexi 573309239 manufacture(0517-0374, 0517-0125) Establishment Name Address ID/FEI Business Operations CENEXI HSC 268155718 manufacture(0517-0381, 0517-0371)