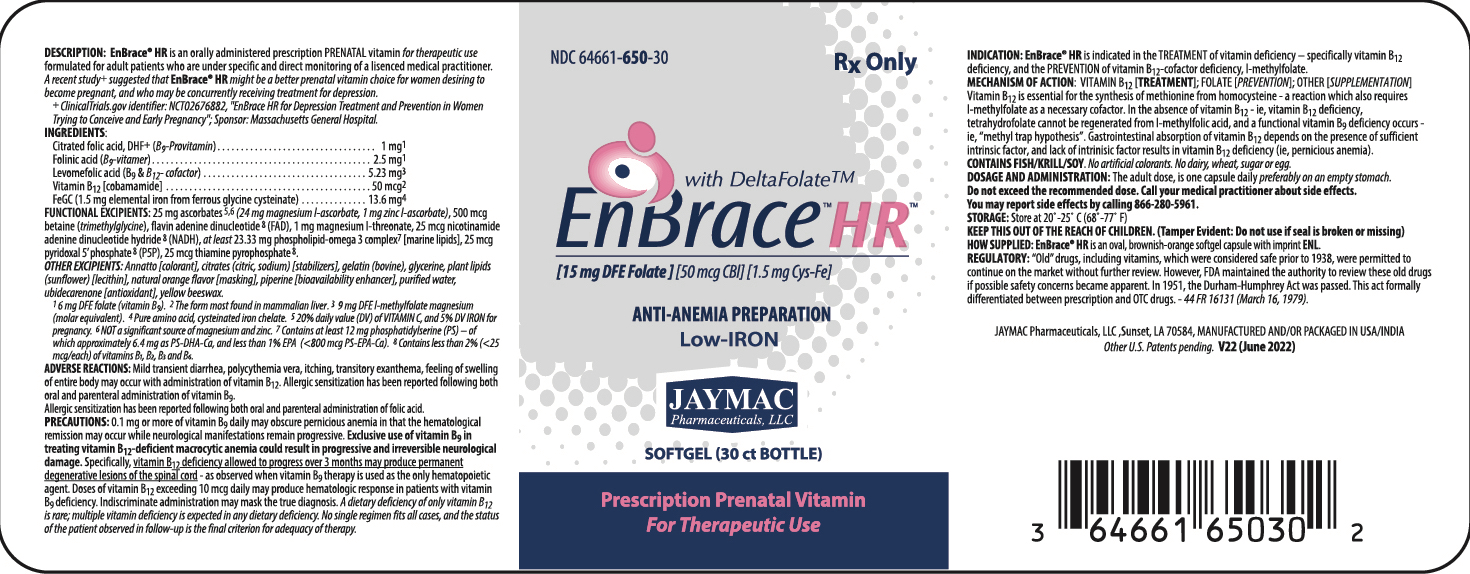
Label: ENBRACE HR- levomefolate magnesium, leucovorin, folic acid, ferrous cysteine glycinate, magnesium ascorbate, zinc ascorbate, cocarboxylase, flavin adenine dinucleotide, nadh, pyridoxal phosphate anhydrous, cobamamide, betaine, magnesium l-threonate, 1,2-docosahexanoyl-sn-glycero-3-phosphoserine calcium, 1,2-icosapentoyl-sn-glycero-3-phosphoserine calcium, and phosphatidyl serine capsule, delayed release pellets
- NDC Code(s): 64661-650-30
- Packager: Jaymac Pharma
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION:
EnBrace® HR is an orally administered prescription prenatal vitamin for therapeutic use formulated for adult patients who are under specific and directr monitoring of a licensed medical practitioner.
EnBrace® HR contains a small amount of iron and may be taken concurrently with iron supplementation.
INGREDIENTS:
Control-release, citrated folic acid, DHF+ (B9 - Provitamin) 1 mg1 Vitamin B12 [cobamamide] 50 mcg2 FeGC [ferrous glycine cysteinate] (1.5 mg elemental iron) 13.6 mg3 ALSO CONTAINS: Folinic acid (B9-vitamer) 2.5 mg1 Levomefolic acid (B9 & B12 - cofactor) 5.23 mg4 1 6 mg DFE folate (vitamin B9)
2 The form most found in mammalian liver - adjusted for stability and pH in the presence of stomach substance, is a cobamamide, or vitamin B12
3 Pure amino acid, cysteinated iron chelate as AminoFerTM* under exclusive license
4 9 mg DFE l-methylfolate magnesium (molar equivalent)
* AminoFerTM (Viva Pharmaceuticals, Canada) U.S. Patent # 7,341,708
-
INACTIVE INGREDIENT
FUNCTIONAL EXCIPIENTS: 25 mg ascorbates5,6 (24 mg magnesium l-ascorbate, 1 mg zinc l-ascorbate) [antioxidant], at least 23.33 mg phospholipid-omega 3 complex7 [marine lipids] , 500 mcg betaine (trimethylglycine) [acidifier], 1 mg magnesium l-threonate [stabilizer].
OTHER EXCIPIENTS: "Annatto color" (as a blend of annatto with stomach substance [thickener/stabilizer]) [colorant], citrates (citric, sodium) [stabilizers], flavin adenine dinucleotide 8 (FAD), gelatin (bovine), glycerine, plant lipids (sunflower) [lecithin], natural orange flavor [masking], nicotinamide adenine dinucleotide hydride 8 (NADH), pyridoxal 5’ phosphate 8 (P5P), piperine [bioavailability enhancer], purified water, thiamine pyrophosphate 8 , ubidecarenone [antioxidant], yellow beeswax.
5 20% daily value (DV) of VITAMIN C, and 5% DV IRON for pregnancy
6 NOT a significant source of magnesium and zinc
7 Contains at least 12 mg phosphatidylserine (PS) – of which approximately 6.4 mg as PS-DHA-Ca, and less than 1% EPA (<800 mcg PS-EPA-Ca)
8 Contains less than 2% (<25 mcg/each) of vitamins B1, B2, B3 and B6
CONTAINS FISH/KRILL/SOY.
Certified 3rd-partyGLUTEN-FREE.No artificial colorants. No dairy, wheat, sugar or egg.
- MECHANISM OF ACTION:
-
iNDICATIONS:
EnBrace® HR is indicated in the TREATMENT of vitamin deficiency - specifically vitamin B12 deficiency, and the PREVENTION of vitamin B12-cofactor deficiency, l-methylfolate.
Requirements of vitamin B9 and/or vitamin B12 in excess of normal due to pregnancy can usually be met with oral supplementation.
-
PRECAUTIONS:
GENERAL:
0.1 mg or more of folic acid daily may obscure pernicious anemia in that the hematological remission may occur while neurological manifestations remain progressive. The safe tolerable limit for folic acid (in preparations) is 1 mg [emphasis added].
Folic acid is not a substitute for vitamin B12 - although it may improve vitamin B12-deficient megaloblastic anemia. Exclusive use of folic acid in treating vitamin B12-deficient megaloblastic anemia could result in progressive and irreversible neurologic damage. Specifically, vitamin B12 deficiency allowed to progress over 3 months may produce permanent degenerative lesions of the spinal cord - as observed when folate therapy is used as the only hematopoietic agent.
Doses of vitamin B12 exceeding 10 mcg daily may produce hematologic response in patients with folate deficiency. Indiscriminate administration may mask the true diagnosis.
A dietary deficiency of only vitamin B12 is rare; multiple vitamin deficiency is expected in any dietary deficiency. No single regimen fits all cases, and the status of the patient observed in follow-up is the final criterion for adequacy of therapy.
DRUG INTERACTIONS:
Colchicine, para-aminosalicylic acid, and heavy alcohol intake for longer than 2 weeks may produce malabsorption of vitamin B12.
CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY:
There is no evidence from long-term use in patients with pernicious anemia that vitamin B12 or folate is carcinogenic. Pernicious anemia is associated with increased incidence of carcinoma of the stomach, but this is believed to be related to the underlying pathology and not to treatment with vitamin B12.
PREGNANCY, NURSING MOTHERS, PEDIATRIC USE:
Vitamin B9 and vitamin B12 are essential vitamins and requirements are increased during pregnancy. Amounts of vitamin B9 and vitamin B12 that are recommended by the Food and Nutrition Board, National Academy of Science - National Research Council for lactating women should be consumed during pregnancy.
Vitamin B12 and vitamin B9 appear in the milk of nursing mothers in concentrations which approximate the mother’s vitamin B12 and vitamin B9 blood level. Amounts of vitamin B12 that are recommended by the Food and Nutrition Board, National Academies of Science - National Research Council for lactating women should be consumed during lactation.
Intake in pediatric patients should be in the amount recommended by the Food and Nutrition Board, National Academy of Science - National Research Council.
- ADVERSE REACTIONS:
-
DOSAGE AND ADMINISTRATION:
The adult dose is one capsule daily preferably on an empty stomach.
As a general rule reticulocyte plasma count, folate and vitamin B12 status must be obtained prior to treatment.
Do not exceed recommended dose. Call your medical practitioner about side effects. You may report side effects by calling 337.662-5962.
- HOW SUPPLIED:
- STORAGE:
-
SPL UNCLASSIFIED SECTION
Rx ONLY
KEEP OUT OF THE REACH OF CHILDREN.
Tamper Evident:Do not use if seal is broken or missing.
MANUFACTURED FOR:
JayMac Pharmaceuticals, LLC; Sunset, LA 70584.
MANUFACTURED AND/OR PACKAGED IN USA/CANADA.
PATENTS:
US other patent applications pending.
TRADEMARKS:
EnBrace® HR is a registered mark of JayMac Pharmaceuticals. DeltaFolate™ is a use-trademark of JayMac Pharmaceuticals.
Revision (Dec 27, 2021)
- PRINCIPAL DISPLAY PANEL:
-
INGREDIENTS AND APPEARANCE
ENBRACE HR
levomefolate magnesium, leucovorin, folic acid, ferrous cysteine glycinate, magnesium ascorbate, zinc ascorbate, cocarboxylase, flavin adenine dinucleotide, nadh, pyridoxal phosphate anhydrous, cobamamide, betaine, magnesium l-threonate, 1,2-docosahexanoyl-sn-glycero-3-phosphoserine calcium, 1,2-icosapentoyl-sn-glycero-3-phosphoserine calcium, and phosphatidyl serine capsule, delayed release pelletsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:64661-650 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOMEFOLATE MAGNESIUM (UNII: 1VZZ62R081) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLIC ACID 5.23 mg LEUCOVORIN (UNII: Q573I9DVLP) (LEUCOVORIN - UNII:Q573I9DVLP) LEUCOVORIN 2.5 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg FERROUS CYSTEINE GLYCINATE (UNII: 8B4OP7RK5N) (FERROUS CATION - UNII:GW89581OWR) FERROUS CYSTEINE GLYCINATE 13.6 mg MAGNESIUM ASCORBATE (UNII: 0N1G678593) (ASCORBIC ACID - UNII:PQ6CK8PD0R) MAGNESIUM ASCORBATE 24 mg ZINC ASCORBATE (UNII: 9TI35313XW) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ZINC ASCORBATE 1 mg COCARBOXYLASE (UNII: Q57971654Y) (COCARBOXYLASE - UNII:Q57971654Y) COCARBOXYLASE 25 ug FLAVIN ADENINE DINUCLEOTIDE (UNII: ZC44YTI8KK) (FLAVIN ADENINE DINUCLEOTIDE - UNII:ZC44YTI8KK) FLAVIN ADENINE DINUCLEOTIDE 25 ug NADH (UNII: 4J24DQ0916) (NADH - UNII:4J24DQ0916) NADH 25 ug PYRIDOXAL PHOSPHATE ANHYDROUS (UNII: F06SGE49M6) (PYRIDOXAL PHOSPHATE ANHYDROUS - UNII:F06SGE49M6) PYRIDOXAL PHOSPHATE ANHYDROUS 25 ug COBAMAMIDE (UNII: F0R1QK73KB) (COBAMAMIDE - UNII:F0R1QK73KB) COBAMAMIDE 50 ug BETAINE (UNII: 3SCV180C9W) (BETAINE - UNII:3SCV180C9W) BETAINE 500 ug MAGNESIUM L-THREONATE (UNII: 1Y26ZZ0OTM) (THREONIC ACID, DL- - UNII:NTD0MI8XRT) MAGNESIUM L-THREONATE 1 mg 1,2-DOCOSAHEXANOYL-SN-GLYCERO-3-PHOSPHOSERINE CALCIUM (UNII: 6WJM73T46K) (1,2-DOCOSAHEXANOYL-SN-GLYCERO-3-PHOSPHOSERINE - UNII:DVY07ILF1W) 1,2-DOCOSAHEXANOYL-SN-GLYCERO-3-PHOSPHOSERINE CALCIUM 6.4 mg 1,2-ICOSAPENTOYL-SN-GLYCERO-3-PHOSPHOSERINE CALCIUM (UNII: 9ABD9DRK7B) (1,2-ICOSAPENTOYL-SN-GLYCERO-3-PHOSPHOSERINE - UNII:C3019D8IIA) 1,2-ICOSAPENTOYL-SN-GLYCERO-3-PHOSPHOSERINE CALCIUM 800 ug PHOSPHATIDYL SERINE (UNII: 394XK0IH40) (PHOSPHATIDYL SERINE - UNII:394XK0IH40) PHOSPHATIDYL SERINE 12 mg Inactive Ingredients Ingredient Name Strength ANNATTO (UNII: 6PQP1V1B6O) GELATIN (UNII: 2G86QN327L) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) PIPERINE (UNII: U71XL721QK) WATER (UNII: 059QF0KO0R) UBIDECARENONE (UNII: EJ27X76M46) YELLOW WAX (UNII: 2ZA36H0S2V) Product Characteristics Color BROWN (ANNATTO) Score no score Shape CAPSULE (oval) Size 14mm Flavor Imprint Code ENL Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64661-650-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/12/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 08/12/2011 Labeler - Jaymac Pharma (830767260) Registrant - Jaymac Pharma (830767260) Establishment Name Address ID/FEI Business Operations Ocean Healthcare Pvt Ltd 873673519 manufacture(64661-650)