Label: GLASSIA- .alpha.1-proteinase inhibitor human injection, solution
- NDC Code(s): 0944-2884-01, 0944-2884-02
- Packager: Takeda Pharmaceuticals America, Inc.
- Category: PLASMA DERIVATIVE
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated September 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GLASSIA safely and effectively. See full prescribing information for GLASSIA.
GLASSIA (Alpha1-Proteinase Inhibitor (Human))
Injection, For Intravenous Use
Initial U.S. Approval: 2010RECENT MAJOR CHANGES
Indications and Usage, Increase serum levels of Alpha1-PI (1) 9/2023 INDICATIONS AND USAGE
GLASSIA is an Alpha1-Proteinase Inhibitor (Human) (Alpha1-PI) indicated for chronic augmentation and maintenance therapy in adults with clinically evident emphysema due to severe hereditary deficiency of Alpha1-PI (alpha1-antitrypsin deficiency). (1)
Limitations of Use:
- The effect of augmentation therapy with any Alpha1-PI, including GLASSIA, on pulmonary exacerbations and on the progression of emphysema in alpha1-antitrypsin deficiency has not been conclusively demonstrated in randomized, controlled clinical trials. (1)
- Clinical data demonstrating the long-term effects of chronic augmentation and maintenance therapy of individuals with GLASSIA are not available. (1)
- GLASSIA is not indicated as therapy for lung disease in patients in whom severe Alpha1-PI deficiency has not been established. (1)
DOSAGE AND ADMINISTRATION
For intravenous use only. (2)
- Dose = 60 mg/kg body weight intravenously once weekly. (2.1)
- Administer at a rate not to exceed 0.2 mL/kg body weight per minute, depending on patient response and comfort. (2.3)
- Dose ranging studies using efficacy endpoints have not been performed. (2.1)
- Always use a 5 micron in-line filter (minimum filter diameter of 32 mm to ensure optimal performance, not supplied) during infusion. (2.3)
- If adverse events occur, reduce the rate or interrupt the infusion until the symptoms subside. Resume the infusion at a rate tolerated by the patient. (2.3)
DOSAGE FORMS AND STRENGTHS
For injection: Approximately 1 gram of functional Alpha1-PI in 50 mL of ready to use solution in a single-dose vial. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Severe hypersensitivity and anaphylactic reactions may occur in IgA deficient patients with antibodies against IgA and in patients with a history of hypersensitivity to other Alpha1-PI products. (5.1)
- May carry a risk of transmitting infectious agents such as viruses, the variant Creutzfeldt-Jakob disease (vCJD) and theoretically, the Creutzfeldt-Jakob disease (CJD) agent. (5.2)
ADVERSE REACTIONS
The most common adverse reactions in clinical trials were headache and upper respiratory infection. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals U.S.A. at 1-877-TAKEDA-7 (1-877-825-3327) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Preparation
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Transmissible Infectious Agents
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
GLASSIA is an Alpha1-Proteinase Inhibitor (Human), indicated for chronic augmentation and maintenance therapy in individuals with clinically evident emphysema due to severe hereditary deficiency of Alpha1-PI, also known as alpha1-antitrypsin (AAT) deficiency.
Limitations of Use:
- The effect of augmentation therapy with GLASSIA or any Alpha1-PI product on pulmonary exacerbations and on the progression of emphysema in Alpha1-PI deficiency has not been conclusively demonstrated in randomized, controlled clinical trials.
- Clinical data demonstrating the long-term effects of chronic augmentation and maintenance therapy of individuals with GLASSIA are not available.
- GLASSIA is not indicated as therapy for lung disease in patients in whom severe Alpha1-PI deficiency has not been established.
-
2 DOSAGE AND ADMINISTRATION
For intravenous use only.
2.1 Dosage
- Administer 60 mg/kg body weight of GLASSIA once weekly by intravenous infusion.
- Dose ranging studies using efficacy endpoints have not been performed.
- The carton and label on each vial of GLASSIA show the actual amount of functionally active Alpha1-PI in milligrams.
2.2 Preparation
- Use aseptic technique.
- Allow the product to reach room temperature prior to infusing and administer within three hours of entering the vials.
- Inspect the vial of GLASSIA. The solution should be clear and colorless to yellow-green and may contain a few protein particles. Discard if the product is cloudy.
- The product is suitable for infusion directly from the vial or pooled into a sterile container for intravenous infusion.
- Use a vented spike (not supplied) to withdraw the solution from the vial.
- Then use a needle (not supplied) to transfer the solution into the intravenous infusion container.
- If using multiple vials to achieve the desired dose, pool GLASSIA into the infusion container by repeating Steps 5 and 6 with a new vented spike and transfer needle for each vial.
2.3 Administration
For intravenous infusion only.
GLASSIA should be administered by a healthcare professional or self-administered by the patient/caregiver after appropriate training. For self-administration, provide the patient/caregiver with detailed instructions and adequate training for infusion in the home or other appropriate setting [see Patient Counseling Information (17)].
- Use aseptic technique.
- Inspect parenteral products visually for particulate matter and discoloration prior to administration whenever solution and container permit.
- Administer GLASSIA alone. Do not mix with other agents or diluting solutions.
- When infusing directly from the vials, use a vented spike (not supplied). If the contents of vials have been pooled to a sterile intravenous container, use an appropriate intravenous administration set.
- Always use a 5 micron in-line filter (minimum filter diameter of 32 mm to ensure optimal performance, not supplied) during infusion.
- Administer GLASSIA within three hours of entering the vials to avoid the potential ill effect of any inadvertent microbial contamination.
- Administer GLASSIA at room temperature through an appropriate intravenous administration set at a rate not to exceed 0.2 mL/kg body weight per minute, and as determined by the response and comfort of the patient. The recommended dosage of 60 mg/kg at a rate of 0.2 mL/kg/min will take approximately 15 minutes to infuse.
- Monitor the infusion rate closely during administration and observe the patient for signs of infusion related reactions. If infusion related adverse reactions occur, reduce the rate or interrupt the infusion as appropriate until the symptoms subside. Resume the infusion at a rate tolerated by the patient, except in the case of severe reaction [see Warnings and Precautions (5.1)].
- Following administration, discard all open vials, unused solution and administration equipment.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
GLASSIA may contain trace amounts of IgA. Patients with known antibodies to IgA, which can be present in patients with selective or severe IgA deficiency, have a greater risk of developing severe hypersensitivity and anaphylactic reactions. Monitor vital signs continuously and observe the patient carefully throughout the infusion. Discontinue the infusion if hypersensitivity symptoms occur and administer appropriate emergency treatment. Have epinephrine and/or other appropriate supportive therapy available for the treatment of any acute anaphylactic or anaphylactoid reaction.
5.2 Transmissible Infectious Agents
Because this product is made from human plasma, it may carry a risk of transmitting infectious agents, such as viruses, the variant Creutzfeldt-Jakob disease (vCJD) and theoretically, the Creutzfeldt-Jakob disease (CJD) agent. This also applies to unknown or emerging viruses and other pathogens. The risk of transmitting an infectious agent has been minimized by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections and by inactivating and removing certain viruses during the manufacturing process. Despite these measures, such products may still potentially transmit human pathogenic agents.
All infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Takeda Pharmaceuticals U.S.A. at 1-877-TAKEDA-7 (1-877-825-3327) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
No seroconversions for hepatitis B or C (HBV or HCV) or human immunodeficiency virus (HIV) or any other known infectious agent were reported with the use of GLASSIA during the clinical trials.
-
6 ADVERSE REACTIONS
The serious adverse reaction observed during clinical trials with GLASSIA was exacerbation of chronic obstructive pulmonary disease (COPD).
The most common adverse reactions during clinical trials were headache and upper respiratory infection.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety profile of GLASSIA was evaluated in a randomized, double-blind, active-control trial and an open-label, non-parallel, dose-escalation trial, in 65 subjects with pre-augmentation therapy serum Alpha1-PI levels less than 11 microM. In the randomized, double-blind, active-control trial, 50 subjects received weekly infusions of GLASSIA or the comparator Alpha1-PI product, Prolastin, at a dosage of 60 mg/kg for a total of 12 doses after which all subjects remaining in the trial were treated for another 12 weeks with GLASSIA only. Overall, 17 subjects received 12 doses and 32 subjects received 22 - 24 doses of GLASSIA during the trial. (One subject randomized to the comparator Alpha1-PI product did not receive any treatment with GLASSIA during the last 12 weeks of the trial.). In the open label, non-parallel, dose-escalation trial, 18 subjects received a single infusion of GLASSIA at dosages of 30, 60 or 120 mg/kg. The population treated in the two trials was 40-74 years old, 54% male, 100% Caucasian.
Tables 1 and 2 compare the adverse reactions reported during the initial 12 weeks (double-blind portion) of the randomized, active comparator trial in all subjects treated with GLASSIA with reactions in the concurrent Prolastin control group. Table 3 compares the frequency of adverse reactions as a percentage of all infusions for GLASSIA and Prolastin-treated subjects over the entire trial period.
Table 1: Number of Subjects/Infusions/Adverse Reactions* Occurring during the First 12 Weeks of Treatment Assessed Parameters GLASSIA Prolastin - *
- An adverse reaction is any adverse event which met any of the following criteria: (a) an adverse event that began within 72 hours following the end of product infusion, or (b) an adverse event considered by either the investigator or sponsor to be at least possibly related to product administration, or (c) an adverse event for which causality assessment was missing or indeterminate.
No. of subjects treated 33 17 No. of infusions 393 190 No. of subjects with serious adverse reactions* (%) 1 (3%) 1 (6%) No. of subjects experiencing an adverse reaction* (%) 22 (67%) 15 (88%) No. of adverse reactions* 47 39 Table 2: Adverse Reactions* Occurring in > 5% of Subjects during the First 12 Weeks of Treatment Adverse Reactions (AR) GLASSIA (N=33)
No. of subjects with adverse reactions* (AR)
(percentage of all subjects)Prolastin (N=17)
No. of subjects with adverse reactions* (AR)
(percentage of all subjects)- *
- An adverse reaction is any adverse event which met any of the following criteria: (a) an adverse event that began within 72 hours following the end of product infusion, or (b) an adverse event considered by either the investigator or sponsor to be at least possibly related to product administration, or (c) an adverse event for which causality assessment was missing or indeterminate.
Cough 3 (9%) 4 (24%) Headache 3 (9%) 3 (18%) Sinusitis 3 (9%) 1 (6%) Upper respiratory tract infection 3 (9%) 0 (0%) Chest discomfort 2 (6%) 0 (0%) Dizziness 2 (6%) 0 (0%) Hepatic enzyme increased 2 (6%) 0 (0%) Table 3: Adverse Reactions* Frequency as a % of all Infusions (≥ 0.5%) Adverse Reactions (AR) GLASSIA†
(Ni=960)
No. of adverse reactions* (AR)
(percentage of all infusions)Prolastin‡
(Ni=190)
No. of adverse reactions* (AR)
(percentage of all infusions)Ni=number of infusions. - *
- An adverse reaction is any adverse event which met any of the following criteria: (a) an adverse event that began within 72 hours following the end of product infusion, or (b) an adverse event considered by either the investigator or sponsor to be at least possibly related to product administration, or (c) an adverse event for which causality assessment was missing or indeterminate.
- †
- Throughout entire 24-week double-blind plus open-label trial period.
- ‡
- Throughout initial 12-week double-blind period.
Upper respiratory tract infection 8 (0.8%) 0 (0.0%) Headache 6 (0.6%) 3 (1.6%) Nasopharyngitis 5 (0.5%) 0 (0%) Chronic Obstructive Pulmonary Disease (COPD) Exacerbations
During the 12-week double blind portion of the randomized, active comparator trial, 4 subjects (12%) had a total of 7 exacerbations of chronic obstructive pulmonary disease (COPD) during GLASSIA treatment and 5 subjects (29%) had a total of 6 exacerbations of COPD during Prolastin treatment. Seventeen additional exacerbations in 14 subjects (28%) occurred during the 12-week open-label treatment period with GLASSIA. The overall rate of pulmonary exacerbations during treatment with either product was 1.3 exacerbations per subject per year. Testing for viral markers for HBV, HCV, HIV-1 and HIV-2 showed no seroconversions during either trial.
Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to GLASSIA with the incidence of antibodies to other products may be misleading.
In the double blind, randomized, active comparator trial of GLASSIA, low level anti-GLASSIA antibodies were detected in one subject at one time point (Week 12) and returned to negative at the end of the study (Week 24) despite continuous exposure to GLASSIA. No immune system adverse reactions were reported.
Immunogenicity was further evaluated in the multicenter study (post-licensure) in subjects with Alpha1-PI deficiency. Of the 34 subjects studied, 2 (6%) developed neutralizing anti-Alpha1-PI antibodies. There was no association between patients with Alpha1-PI antibodies and immune-mediated treatment emergent adverse reactions or associated decrease in plasma Alpha1-PI levels.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of GLASSIA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Respiratory, Thoracic, and Mediastinal Disorders: Dyspnea
Gastrointestinal Disorders: Nausea
General Disorders and Administration Site Conditions: Fatigue
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with GLASSIA use in pregnant women to inform a drug-associated risk. Animal reproduction studies have not been conducted with GLASSIA. It is also not known whether GLASSIA can cause fetal harm when administered to pregnant women or can affect reproductive capacity. GLASSIA should be given to a pregnant woman only if clearly needed.
8.2 Lactation
Risk Summary
There is no information regarding the presence of GLASSIA in human milk, the effect on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for GLASSIA and any potential adverse effects on the breastfed infant from GLASSIA.
8.5 Geriatric Use
Clinical trials of GLASSIA included 11 subjects of 65 years of age or older. This number of subjects was not sufficient to determine whether they respond differently from younger subjects. As for all patients, dosing for geriatric patients should be appropriate to their overall situation. Safety and effectiveness in patients over 65 years of age have not been established.
-
11 DESCRIPTION
GLASSIA is a sterile, ready to use, liquid preparation of purified human alpha1-proteinase inhibitor (Alpha1-PI), also known as alpha1-antitrypsin (AAT). The solution contains 2% active Alpha1-PI in a phosphate-buffered saline solution. The specific activity of GLASSIA is ≥ 0.7 mg functional Alpha1-PI per mg of total protein. Not less than 90% of the Alpha1-PI in GLASSIA is of the monomeric form as measured by size-exclusion chromatography.
GLASSIA is prepared from human plasma obtained from US-licensed plasma collection centers by a modified version of the cold ethanol fractionation process and the Alpha1-PI is then purified using chromatographic methods.
Individual plasma units used for production of GLASSIA are tested using FDA- licensed serological assays for hepatitis B surface antigen (HBsAg) and for antibodies to hepatitis C virus (HCV) and human immunodeficiency virus types 1 and 2 (HIV-1/2), as well as by FDA-licensed Nucleic Acid Testing (NAT) for HCV and HIV-1. Each plasma unit must be non-reactive (negative) in all tests. Plasma is also tested by in-process NAT procedures for parvovirus B19 and the limit for B19 DNA in the manufacturing pool is set not to exceed 104 IU per mL.
To reduce the risk of viral transmission, the manufacturing process for GLASSIA includes two steps specifically designed to remove or inactivate viruses. The first of these is nanofiltration (NF) through a 15 nm filter which can remove both enveloped and non–enveloped viral agents and the second is solvent/detergent (S/D) treatment with a mixture of tri-(n-butyl) phosphate (TNBP) and Polysorbate 80 (Tween 80) which inactivates enveloped viral agents such as HIV, HBV and HCV.
The effectiveness of the S/D treatment and nanofiltration procedures for reducing virus content has been assessed using a series of viruses with a range of physico-chemical characteristics. The results of the viral challenge studies are summarized in Table 4.
Table 4: Log10 Virus Reduction during Manufacture of GLASSIA Process Step Enveloped Viruses HIV-1 Enveloped Viruses PRV Enveloped Viruses BVDV Enveloped Viruses WNV Non-enveloped Viruses HAV Non-enveloped Viruses PPV N/A - Not Applicable. The S/D treatment is not relevant for non-enveloped viruses.
ND - Not Done
Nanofiltration > 5.59 > 5.57 > 5.74 ND > 4.99 4.04 S/D treatment > 6.41 > 6.14 > 5.61 > 6.32 N/A N/A Global Reduction Factor > 12.00 > 11.71 > 11.35 > 6.32 > 4.99 4.04 HIV-1 Human immunodeficiency virus Type 1 WNV West Nile virus PRV Pseudorabies virus HAV Hepatitis A virus BVDV Bovine viral diarrhea virus PPV Porcine parvovirus -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
GLASSIA administration is intended to inhibit serine proteases such as neutrophil elastase (NE), which is capable of degrading protein components of the alveolar walls and which is chronically present in the lung.
Alpha1-PI deficiency is a chronic, autosomal, co-dominant hereditary disorder characterized by reduced levels of Alpha1-PI in the blood and lungs1, 2. Smoking is an important risk factor for the development of emphysema in patients with Alpha1-PI deficiency3. Because emphysema affects many, but not all individuals with the more severe genetic variants of Alpha1-PI deficiency (AAT deficiency), augmentation therapy with Alpha1-Proteinase Inhibitor (Human) is indicated only in patients with severe Alpha1-PI deficiency who have clinically evident emphysema.
A large number of phenotypic variants of Alpha1-PI deficiency exist, not all of which are associated with the clinical disease. Approximately 95% of identified Alpha1-PI deficient individuals have the PiZZ variant, typically characterized by Alpha1-PI serum levels < 35% of normal. Individuals with the Pi(null)(null) variant have no Alpha1-PI protein in their serum2, 3. Individuals with the lack of, or low, endogenous serum levels of Alpha1-PI, i.e., below 11 μM, manifest a significantly increased risk for development of emphysema above the general population background risk4, 5. In addition, PiSZ individuals, whose serum Alpha1-PI levels range from approximately 9 to 23 μM are considered to have moderately increased risk for developing emphysema, regardless of whether their serum Alpha1-PI levels are above or below 11 μM6.
Augmenting the levels of functional protease inhibitor by intravenous infusion is an approach to therapy for patients with Alpha1-PI deficiency. However, the efficacy of augmentation therapy in affecting the progression of emphysema has not been demonstrated in randomized, controlled clinical trials. The intended theoretical goal is to provide protection to the lower respiratory tract by correcting the imbalance between neutrophil elastase and protease inhibitors. Whether augmentation therapy with GLASSIA or any Alpha1-PI product actually protects the lower respiratory tract from progressive emphysematous changes has not been conclusively demonstrated in adequately powered, randomized controlled clinical trials. Although the maintenance of blood serum levels of Alpha1-PI (antigenically measured) above 11 μM has been historically postulated to provide therapeutically relevant anti-neutrophil elastase protection, this has not been proven. Individuals with severe Alpha1-PI deficiency have been shown to have increased neutrophil and neutrophil elastase concentrations in lung epithelial lining fluid compared to normal PiMM individuals, and some PiSZ individuals with Alpha1-PI above 11 μM have emphysema attributed to Alpha1-PI deficiency. These observations underscore the uncertainty regarding the appropriate therapeutic target serum level of Alpha1-PI during augmentation therapy.
12.2 Pharmacodynamics
Administration of GLASSIA to patients with Alpha1-PI deficiency augments the level of the deficient protein. Normal individuals have levels of Alpha1-PI greater than 22 μM. The clinical benefit of the increased blood levels of Alpha1-PI at the recommended dose has not been established.
The clinical efficacy of GLASSIA in influencing the course of pulmonary emphysema or the frequency, duration, or severity of pulmonary exacerbations has not been demonstrated in randomized, controlled clinical trials.
GLASSIA increases antigenic and functional (anti-neutrophil elastase capacity, ANEC) levels of Alpha1-PI in both the serum and the lung epithelial lining fluid (ELF) [see Clinical Studies (14)].
12.3 Pharmacokinetics
A prospective, open-label, uncontrolled multicenter pharmacokinetic trial was conducted in 7 females and 11 males with congenital Alpha1-PI deficiency, ranging in age from 40 to 69 years. Subjects received a single dose of GLASSIA either 30 mg/kg, 60 mg/kg or 120 mg/kg. Blood samples for pharmacokinetic study were taken prior to and within 5 minutes of completion of the infusion, and then at 1 hour, 6 hours, 12 hours, 24 hours, 3 days and 7 days.
The mean results for pharmacokinetic parameters in the 60 mg/kg dosage group are shown in Table 5. The pharmacokinetics of GLASSIA were linear over the dose range of 30-120 mg/kg.
Table 5: Pharmacokinetic Parameters for Functional Alpha1-PI (Dosage 60 mg/kg; n=6) - *
- Any assessment of the clinical relevance of half-life in this trial should be viewed with caution, due to the short duration of blood sampling.
Pharmacokinetic Parameter 60 mg/kg
Dose GroupTerminal Half-Life (h) * 111 ± 33 Area under the curve(0-168 h) (mg∙h/mL) 89 ± 10 Clearance (mL/h/kg) 0.68 ± 0.1 Volume of Distribution (L) 3.2 ± 0.3 -
14 CLINICAL STUDIES
A randomized, double-blind trial with a partial cross-over was conducted to compare GLASSIA to a commercially available preparation of Alpha1-PI (Prolastin) in 50 Alpha1-PI-deficient subjects. The trial objectives were to demonstrate that the pharmacokinetics of antigenic and/or functional Alpha1-PI in GLASSIA were not inferior to those of the control product, to determine whether GLASSIA maintained antigenic and/or functional plasma levels of at least 11 microM (57 mg/dL) and to compare Alpha1-PI trough levels (antigenic and functional) over 6 infusions.
For inclusion in the trial, subjects were required to have lung disease related to Alpha1-PI deficiency and 'at-risk' alleles associated with Alpha1-PI plasma levels < 11 microM. Subjects already receiving Alpha1-PI therapy were required to undergo a 5-week wash-out period of exogenous Alpha1-PI prior to dosing.
Fifty subjects received either GLASSIA (33 subjects) or the comparator product (17 subjects) at a dose of 60 mg/kg intravenously per week for 12 consecutive weeks. From Week 13 to Week 24 all subjects received open-label weekly infusions of GLASSIA at a dose of 60 mg/kg.
Trough levels of functional and antigenic Alpha1-PI were measured prior to treatment, at baseline and throughout the trial until Week 24. The median trough Alpha1-PI values for Weeks 7-12 for subjects receiving GLASSIA were 14.5 microM (range: 11.6 to 18.5 microM) for antigenic and 11.8 microM (range: 8.2 to 16.9 microM) for functional Alpha1-PI. Eleven of 33 subjects (33.3%) receiving GLASSIA had mean steady-state functional Alpha1-PI levels below 11 microM. GLASSIA was shown to be non-inferior to the comparator product.
Serum Alpha1-PI trough levels rose substantially in all subjects by Week 2 and were comparatively stable during Weeks 7 to 12. All subjects receiving GLASSIA had mean serum trough antigenic Alpha1-PI levels greater than 11 microM during Weeks 7-12.
A subset of subjects in both treatment groups (n = 7 for subjects receiving GLASSIA) underwent broncho-alveolar lavage (BAL) and were shown to have increased levels of antigenic Alpha1-PI and Alpha1-PI - neutrophil elastase complexes in the epithelial lining fluid at Week 10-12 over levels found at baseline, demonstrating the ability of the product to reach the lung.
The clinical efficacy of GLASSIA in influencing the course of pulmonary emphysema or the frequency, duration, or severity of pulmonary exacerbations has not been demonstrated in randomized, controlled clinical trials.
A prospective, randomized, double-blind, active-controlled, crossover trial was conducted in thirty healthy adult subjects (23 [77%] male and 7 [23%] female; median age of 24 years [range: 19 to 61 years]), each receiving 2 infusions of GLASSIA at a dosage of 60 mg/kg. The objective of the trial was to assess the safety and tolerability of GLASSIA at an intravenous infusion rate of 0.2 mL/kg/min. On Day 1, 15 subjects received GLASSIA at 0.04 mL/kg/min with a simultaneous administration of placebo (2.5% human albumin in normal saline, for the purpose of masking infusion) at 0.2 mL/kg/min (Cohort 1), and 15 subjects received GLASSIA at 0.2 mL/kg/min with a simultaneous administration of placebo at 0.04 mL/kg/min (Cohort 2). Two weeks later (Day 15), the 15 subjects in Cohort 1 received the second infusion of GLASSIA at 0.2 mL/kg/min with a simultaneous administration of placebo at 0.04 mL/kg/min, and the 15 subjects in Cohort 2 received GLASSIA at 0.04 mL/kg/min with a simultaneous administration of placebo at 0.2 mL/kg/min. Neither efficacy nor exposure (antigenic or functional AAT) was measured in this trial.
The safety, immunogenicity, and effects on the Alpha1-PI levels in epithelial lining fluid (ELF) following GLASSIA therapy were evaluated in a multicenter study (post-licensure) of 34 subjects with emphysema due to congenital A1PI deficiency. Subjects received GLASSIA at a dosage of 60 mg/kg for 25 weeks. Sixteen subjects underwent bronchoalveolar lavage (BAL) at baseline and at Weeks 12-14. GLASSIA augmentation therapy resulted in a statistically significant increase in antigenic Alpha1-PI levels in ELF (median change=0.5 μM; geometric mean ratio=5.4, p<0.001; Table 6). Functional Alpha1-PI levels in ELF also showed a statistically significant increase in response to GLASSIA (median change=0.3 μM, geometric mean ratio=2.3, p<0.001; Table 6).
Table 6: Summary and Analysis of Change from Baseline in Antigenic and Functional Alpha1-PI Levels in the Epithelial Lining Fluid Alpha1-PI Levels
(n = 16)Geometric Mean*
Baseline BAL
(µM)Geometric Mean* On-Treatment BAL (µM) (Weeks 12-14) Geometric Mean Ratio*
(p-value)- *
- From a mixed-effects model with visit as fixed effect and subject as random effect; a 1-sided paired t-test for H0: Diff (On-Treatment BAL – Baseline BAL) ≤ 0 and H1: Diff (On-Treatment BAL – Baseline BAL) > 0.
Antigenic Alpha1-PI
Levels0.1 0.6 5.4 (<0.001) Functional Alpha1-PI
Levels0.2 0.5 2.3 (<0.001) -
15 REFERENCES
- WHO. "α1-Antitrypsin deficiency." Memorandum from a WHO meeting. 1997;75: 397-415.
- Stoller JK, et al. Augmentation therapy with α1-antitrypsin: patterns of use and adverse events. Chest 2003; 123:1425-34.
- Cox DW. α1-Antitrypsin Deficiency. In: Scriver C, Sly W, eds. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw Hill; 2002:5559-78.
- Crystal RG, et al. The alpha1-antitrypsin gene and its mutations. Clinical consequences and strategies for therapy. Chest 1989;95:196-208.
- Crystal RG. α1-Antitrypsin deficiency: pathogenesis and treatment. Hosp Pract (Off Ed) 1991;26:81-4, 8-9, 93-4.
- Turino GM, et al. Clinical features of individuals with PI*SZ phenotype of α1-antitrypsin deficiency. Am J Respir Crit Care Med 1996;154:1718-25.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
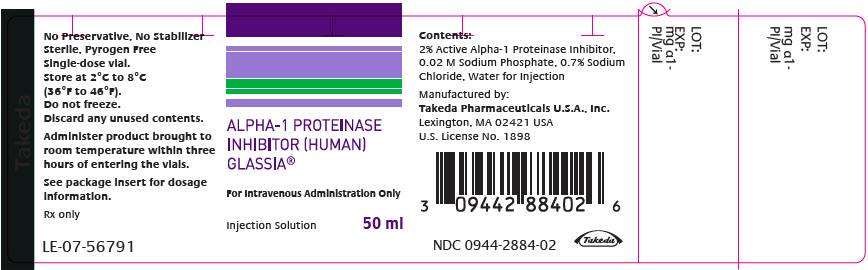
Each carton of GLASSIA contains a single-dose vial containing approximately 1 gram of functional Alpha1-PI in 50 mL of solution (NDC 0944-2884-01).
- Store GLASSIA at 2°C to 8°C (36°F to 46°F). Do not freeze.
- Product may be stored at room temperatures not exceeding 25°C (77°F) for up to one month.
- Once removed from refrigeration use within one month.
- Keep vial in carton until required for use.
- Do not use after the expiration date printed on the label.
- GLASSIA contains no preservatives and no latex.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Detailed Instructions for Administration).
- Inform patients of the early signs of hypersensitivity reactions, including hives, generalized urticaria, chest tightness, dyspnea, wheezing, faintness, hypotension, and anaphylaxis. Advise patients to discontinue use of the product and contact their physician and/or seek immediate emergency care, depending on the severity of the reaction, if these symptoms occur.
- Inform patients that GLASSIA is made from human plasma and may contain infectious agents that can cause disease (e.g., viruses and, theoretically, the CJD agent). Explain that the risk of GLASSIA transmitting an infectious agent has been reduced by screening the plasma donors, by testing the donated plasma for certain virus infections, and by a process demonstrated to inactivate and/or remove certain viruses during manufacturing [see Warnings and Precautions (5.2)]. Symptoms of a possible virus infection include headache, fever, nausea, vomiting, weakness, malaise, diarrhea, or, in the case of hepatitis, jaundice.
- Inform patients that administration of GLASSIA has been demonstrated to raise the plasma level of Alpha1-PI, but that the effect of this augmentation on the frequency of pulmonary exacerbations and on the rate of progression of emphysema has not been established by clinical trials.
Self-administration – If self-administration is deemed appropriate, ensure that the patient/caregiver receives detailed instructions and adequate training on how to administer in the home or other appropriate setting and has demonstrated the ability to independently administer GLASSIA.
- Ensure the patient/caregiver understands the importance of weekly infusions to raise the plasma level of Alpha1-PI.
- Ensure that the patient/caregiver has access to and has received training in the administration of subcutaneous epinephrine and/or other appropriate supportive therapy for the treatment of any acute anaphylactic or anaphylactoid reaction.
- Advise the patient/caregiver to report any adverse reactions or problems following GLASSIA administration to their physician or healthcare provider.
- Instruct the patient/caregiver to keep a treatment infusion log. This infusion log should include information such as, the lot number, the time, date, and any reactions.
©2023 Takeda Pharmaceutical Company Limited. All rights reserved. GLASSIA is a registered trademark of Kamada Ltd., and used under license.
Takeda® and the Takeda Logo® are registered trademarks of Takeda Pharmaceutical Company Limited.
Manufactured by:
Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
USA
U.S. License No. 1898
GLA378
-
PATIENT PACKAGE INSERT
Patient Information
GLASSIA
(Alpha1-Proteinase Inhibitor (Human)) Injection,
For Intravenous UseThe following summarizes important information about GLASSIA (pronounced glass-see-ă). Please read it carefully before using this medicine. This information does not take the place of talking with your healthcare professional, and it does not include all of the important information about GLASSIA. If you have any questions after reading this, ask your healthcare professional.
What is GLASSIA?
GLASSIA is a liquid medicine containing human Alpha1-Proteinase Inhibitor (Alpha1-PI) also known as alpha1-antitrypsin (AAT), which is purified from human blood. The main purpose of infusing GLASSIA is to increase the levels of the AAT protein in your blood and lungs. AAT protein protects the lung tissue by blocking certain enzyme-caused damage. Such damage can lead to severe lung disease, such as emphysema.
Limitations of Use:
- The effects of increasing the AAT protein levels with GLASSIA or any other Alpha1-PI product on worsening pulmonary function and progression of emphysema have not been proven in clinical trials.
- The long-term effects of AAT replacement and maintenance therapy with GLASSIA have not been studied.
- GLASSIA is not intended as a therapy in individuals with lung disease other than severe Alpha1-PI deficiency.
Who should not take GLASSIA?
You should not use GLASSIA if you:
- Have IgA deficiency with antibodies to IgA
- Have had a severe allergic reaction to human Alpha1-PI products
What is the most important information that I should know about GLASSIA?
Severe allergic reactions can occur with GLASSIA. Your doctor will inform you about signs of allergic reactions which include hives, swelling in the mouth or throat, itching, tightness in the chest, trouble breathing, wheezing, faintness, low blood pressure, or serious allergic reaction. If you have any of these reactions, discontinue use of the product and contact your physician and/or seek immediate emergency care, depending on the severity of the reaction.
If you or your caregiver will be administering GLASSIA outside a healthcare setting, ask your doctor about an epinephrine pen and/or other supportive care for certain severe allergic reactions. Ask your doctor to make sure you receive training on how and when to use any prescribed supportive care medicine and keep it close at hand when administering GLASSIA.
How should I take GLASSIA?
- GLASSIA is given directly into the bloodstream.
- You can get GLASSIA at your healthcare professional's office, clinic, hospital, or delivered directly to your home by a healthcare professional from a limited network of specialty pharmacy providers.
- Your healthcare professional will decide if self-infusion in your home is right for you. You should be trained on how to do infusions by your healthcare professional.
What should I tell my healthcare professional before I start using GLASSIA?
Before starting GLASSIA, tell your healthcare professional if you:
- Have IgA deficiency with antibodies to IgA.
- Have a history of severe allergic reactions to Alpha1-PI products.
What are the possible or reasonably likely side effects of GLASSIA?
- A possible side effect to GLASSIA is worsening or flare-up of your chronic obstructive pulmonary disease (COPD) in which your breathing gets worse than usual.
- Call your healthcare professional or go to your emergency department right away if you get: Hives, swelling in the mouth or throat, itching, chest tightness, trouble breathing, wheezing, fainting or dizziness. These could be signs of a serious allergic reaction.
- The most common side effects are headache and upper respiratory tract infections. Other possible side effects of GLASSIA include: cough, sinus infection, chest discomfort, dizziness, increased liver enzymes, shortness of breath, nausea, and fatigue.
These are not all of the possible side effects for GLASSIA. You can ask your healthcare professional for information that is provided to healthcare professionals. Talk to your healthcare professional about any side effects that bother you or that don't go away.
How do I store GLASSIA?
Store GLASSIA refrigerated or at room temperature.
- You can store GLASSIA in the refrigerator (36°F to 46°F [2°C to 8°C]). Do not freeze.
- You can store GLASSIA at room temperature (up to 77°F [25°C]) for up to one month. You must use GLASSIA within one month once you remove it from the refrigerator. Do not re-refrigerate GLASSIA once the product has been stored at room temperature.
- Keep the GLASSIA vial in the box until you are ready to administer the product.
Check the expiration date on the carton and vial label. Do not use GLASSIA after the expiration date.
©2023 Takeda Pharmaceutical Company Limited. All rights reserved.
GLASSIA is a registered trademark of Kamada Ltd., and used under license.
Takeda® and the Takeda Logo® are registered trademarks of Takeda Pharmaceutical Company Limited.
Manufactured by:
Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
USA
U.S. License No. 1898
Revised: September 2023 GLA378
-
INSTRUCTIONS FOR USE
(Alpha1-Proteinase Inhibitor (Human)) Injection,
For Intravenous Use
GLASSIA
Detailed Instructions For AdministrationDo not attempt to do an infusion to yourself unless you or your caregiver have been taught how by your healthcare professional.
Always follow the specific instructions given by your healthcare professional. The steps listed below are general guidelines for using GLASSIA. If you are unsure of the procedures, call your healthcare professional before using.
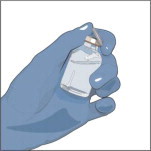
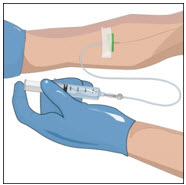
Prepare a clean flat surface and gather all the materials you will need for the infusion. Check the expiration date, and let the vial of GLASSIA warm up to room temperature. Do not apply heat, place in hot water, or microwave. Wash your hands and put on clean exam gloves. If you are infusing yourself at home, the use of gloves is optional.
- 1.
-
Check the vial(s) of GLASSIA:
- Do not use if the protective cap is missing or broken.
- Look at the color: it should be clear and colorless to yellow-green.
- Do not use if the solution is cloudy.
- It may contain a few (protein) particles.
- Do not shake the vial(s).
- 2.
-
Gather all supplies
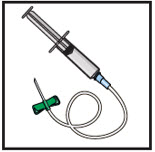
- Gather all supplies: vial(s) of GLASSIA as prescribed and infusion supplies (not provided): tourniquet, alcohol swabs, intravenous needle set (butterfly), vented spike(s), 5 micron in-line filter (minimum of 32 mm diameter is recommended for optimal performance), 60 mL sterile syringe(s), transfer needle(s), sterile intravenous infusion container (bag) (if necessary), administration infusion set, extension set (if necessary), bandage, tape, sterile gauze, sharps container, IV pole or hook, clean gloves (if necessary) and infusion log.
- If a drip chamber is used it must not contain a filter.
- If your doctor has prescribed epinephrine pen and/or other supportive care for certain severe allergic symptoms, keep it close at hand during your infusion. Carefully follow your doctor's instructions and training if you have to administer the prescribed medicine for a severe allergic reaction.
- Wash your hands and allow them to dry.
- Apply gloves as directed by your healthcare professional.
Open supplies as shown by your healthcare professional.
- 3.
-
Prepare the vials(s) for infusion as directed by your healthcare professional:
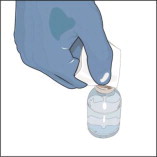
- Remove the protective cap from the vial.
- Wipe the stoppers of each vial that you will need for your dose with a sterile alcohol swab and allow the stopper to dry.
- As directed by your healthcare professional, you may infuse directly from the vial or pool the recommended number of vials of GLASSIA into an empty, sterile container (bag) for intravenous infusion. Use the product within 3 hours of entering the vial(s) or pooling into a sterile container (bag).
If you are pooling into a sterile container (bag):
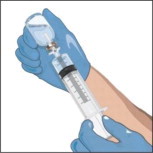
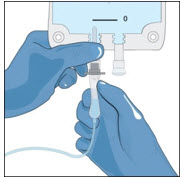
- Attach a vented spike to a sterile syringe.
- Insert the vented spike into the center of the GLASSIA vial.
- Turn the vial upside down and pull back on the plunger to pull the GLASSIA solution into the syringe(s).
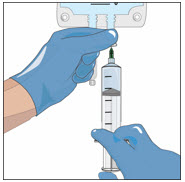
- Twist to remove the syringe from the vented spike.
- Point the syringe tip up and gently push the plunger of the syringe to remove the air.
- Attach a transfer needle to the filled syringe.
- Wipe the injection port on the empty sterile container (bag) with an alcohol swab before each needle insertion. Use a new alcohol swab for each vial.
- Remove protective cover of needle and insert the needle into the injection port and fill the empty bag. Avoid touching the exposed needle.
- Remove the needle from the injection port and discard the syringe and needle in a sharps container.
- Repeat these steps, if using multiple vials to achieve the desired dose as directed by your healthcare professional, using a new vented spike and transfer needle with each vial.
- 4.
-
Prepare the infusion set:
- Close the roller clamp on the IV infusion set.
- Attach an in-line 5 micron filter to the end of the IV infusion set.
- Attach an extension set (if necessary) to in-line filter .
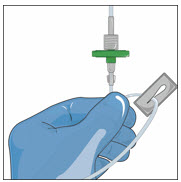
- Remove cap from the IV infusion set spike and remove the cover from the infusion port on the bag containing GLASSIA. Avoid touching the exposed spike or infusion port.
- Insert the spike more than halfway into the infusion port on the containing GLASSIA by twisting and pushing.
- Hang the pooling bag from an IV pole or hook.
- Squeeze the drip chamber until it is half-full and fill the IV tubing with GLASSIA solution by releasing the roller clamp. Close clamp when the solution reaches the end of the extension set.
- 5.
-
Prepare the infusion site(s):
- Connect the saline syringe to the needle set (butterfly) and fill.
- Select an infusion site as directed by your healthcare professional.
- Rotate infusion sites as directed.
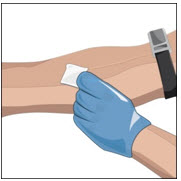
- Apply a tourniquet.
- Get the injection site ready by wiping the skin well with an alcohol swab (or other suitable solution suggested by your healthcare provider) and wait for the skin to dry. Do not touch or blow on the cleaned infusion site.
- 6.
-
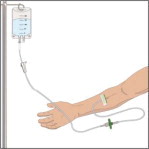
Insert and secure the intravenous needle set as directed by your healthcare professional.
- Release the tourniquet.
- Draw back slightly on syringe to check for blood return. The presence of blood means the needle placement is correct. If there is no blood present, repeat steps.
- Flush the intravenous needle set (butterfly) with normal saline.
- Remove the saline flush syringe and attach the IV infusion set filled with GLASSIA solution.
- 7.
-
Follow your healthcare professional's instructions for infusing GLASSIA:
- Open the roller clamp and administer GLASSIA solution at room temperature at a rate as directed by your healthcare professional. The maximum recommended infusion rate for GLASSIA is 0.2 milliliter per kilogram body weight per minute which will take approximately 15 minutes to infuse.
- Check infusion site occasionally throughout the infusion to make sure that the drug is flowing and there is no bleeding.
- When the infusion is complete, take the needle out of the vein and discard in a sharps container. Use sterile gauze to put pressure on the infusion site for several minutes and then apply a sterile bandage.
- Do not recap the intravenous needle set. Place the needle set in a sharps container for proper disposal. Do not dispose of these supplies in ordinary household trash.
- 8.
-
Record the infusion:
- Write down the product lot number and expiration date in your treatment record/infusion log.
- Write down the date, time (start and end), dose, site(s) of infusion (to assist in rotating sites) and any reactions after each infusion. Report all reactions to your healthcare professional.
- Throw away all open vials, and any unused solution into a sharps container, as recommended by your healthcare professional.
©2023 Takeda Pharmaceutical Company Limited. All rights reserved.
GLASSIA is a registered trademark of Kamada Ltd., and used under license.
Manufactured by:
Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
USA
U.S. License No. 1898
Revised: September 2023 GLA378
- PRINCIPAL DISPLAY PANEL - 50 ml Vial Label
- PRINCIPAL DISPLAY PANEL - 50 ml Vial Carton
-
INGREDIENTS AND APPEARANCE
GLASSIA
.alpha.1-proteinase inhibitor human injection, solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0944-2884 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength .ALPHA.1-PROTEINASE INHIBITOR HUMAN (UNII: F43I396OIS) (.ALPHA.1-PROTEINASE INHIBITOR HUMAN - UNII:F43I396OIS) .ALPHA.1-PROTEINASE INHIBITOR HUMAN 1 g in 50 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-2884-01 1 in 1 CARTON 1 NDC:0944-2884-02 50 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125325 07/01/2010 Labeler - Takeda Pharmaceuticals America, Inc. (039997266) Establishment Name Address ID/FEI Business Operations Baxalta Belgium Manufacturing SA 370634700 MANUFACTURE(0944-2884) , LABEL(0944-2884) , PACK(0944-2884) , ANALYSIS(0944-2884) Establishment Name Address ID/FEI Business Operations Kamada - Production Plant 649062486 MANUFACTURE(0944-2884) , LABEL(0944-2884) , PACK(0944-2884) , ANALYSIS(0944-2884)