Label: BENEFIX (coagulation factor ix- recombinant kit
-
NDC Code(s):
0069-1111-01,
58394-041-11,
58394-133-03,
58394-134-03, view more58394-135-03, 58394-136-03, 58394-137-03, 58394-633-03, 58394-634-03, 58394-635-03, 58394-636-03, 58394-637-03
- Packager: Wyeth BioPharma Division of Wyeth Pharmaceuticals LLC
- Category: PLASMA DERIVATIVE
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated March 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BeneFIX safely and effectively. See full prescribing information for BeneFIX.
BeneFIX® [coagulation factor IX (recombinant)]
lyophilized powder for solution, for intravenous use
Initial U.S. Approval: 1997INDICATIONS AND USAGE
BeneFIX is a recombinant human blood coagulation factor IX indicated for adults and children with hemophilia B (congenital factor IX deficiency or Christmas disease) for:
- o
- On-demand treatment and control of bleeding episodes. (1)
- o
- Perioperative management of bleeding. (1)
- o
- Routine prophylaxis to reduce the frequency of bleeding episodes. (1)
Limitations of Use
BeneFIX is not indicated for induction of immune tolerance in patients with hemophilia B. (1)
DOSAGE AND ADMINISTRATION
For intravenous use after reconstitution only.
- •
- One international unit (IU) of BeneFIX per kilogram of body weight increased the circulating activity of factor IX as follows:
- •
- Determine the initial estimated dose using the following formula:
Required units =
body weight (kg) × desired factor IX increase (IU/dL or % of normal) × reciprocal of observed recovery (IU/kg per IU/dL). (2.1)
- •
- Dosage and duration of treatment with BeneFIX depend on the severity of the factor IX deficiency, the location and extent of bleeding, and the patient's clinical condition, age and recovery of factor IX. (2)
- •
- Routine Prophylaxis:
For long-term prophylaxis against bleeding, the recommended regimen is 100 IU/kg once weekly. Adjust the dosing regimen (dose or frequency) based on the patient's clinical response. (2.1)
DOSAGE FORMS AND STRENGTHS
BeneFIX is available as lyophilized powder in single-use vials containing nominally 250, 500, 1000, 2000, or 3000 IU. (3)
CONTRAINDICATIONS
Do not use in patients who have manifested life-threatening, immediate hypersensitivity reactions, including anaphylaxis, to the product or its components, including hamster protein. (4)
WARNINGS AND PRECAUTIONS
- •
- Hypersensitivity reactions, including anaphylaxis, are possible. Should symptoms occur, discontinue treatment with the product and seek emergency treatment. Patients may develop hypersensitivity to hamster (CHO) protein as BeneFIX contains trace amounts. (5.1)
- •
- BeneFIX has been associated with the development of thromboembolic complications, including in patients receiving continuous infusion through a central venous catheter. (5.2)
- •
- Nephrotic syndrome has been reported following immune tolerance induction with factor IX products in hemophilia B patients with factor IX inhibitors and a history of allergic reactions to factor IX. (5.3)
- •
- Development of neutralizing antibodies (inhibitors) to BeneFIX may occur. If expected plasma factor IX activity levels are not attained, or if patient presents with allergic reaction, or if bleeding is not controlled with an expected dose, perform an assay that measures factor IX inhibitor concentration. (5.4)
ADVERSE REACTIONS
The most common adverse reactions (incidence >5%) from clinical trials were fever, cough, nausea, injection site reaction, injection site pain, headache, dizziness and rash. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Wyeth Pharmaceuticals LLC at 1-800-934-5556 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Pediatric Use: Lower recovery, shorter half-life and higher clearance (based on kg body weight) have been observed in children (<12 years). Dose adjustment may be needed. (8.4, 12.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Preparation and Reconstitution
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Thromboembolic Complications
5.3 Nephrotic Syndrome
5.4 Neutralizing Antibodies (Inhibitors)
5.5 Monitoring Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
BeneFIX®, Coagulation Factor IX (Recombinant), is a human blood coagulation factor indicated in adults and children with hemophilia B (congenital factor IX deficiency or Christmas disease) for:
- o
- On-demand treatment and control of bleeding episodes
- o
- Perioperative management of bleeding
- o
- Routine prophylaxis to reduce the frequency of bleeding episodes
Limitation of Use
BeneFIX is not indicated for induction of immune tolerance in patients with hemophilia B [see Warnings and Precautions (5.3)].
-
2 DOSAGE AND ADMINISTRATION
For intravenous use after reconstitution only.
- •
- Each vial of BeneFIX has the recombinant Factor IX (rFIX) potency in the International Units (IU) stated on the vial.
- •
- Initiate treatment under the supervision of a physician experienced in the treatment of hemophilia B.
- •
- Dosage and duration of treatment with BeneFIX depend on the severity of the factor IX deficiency, the location and extent of bleeding, and the patient's clinical condition, age and recovery of factor IX.
- •
- Dosing of BeneFIX may differ from that of plasma-derived factor IX products [see Clinical Pharmacology (12)]. Subjects at the low end of the observed factor IX recovery may require upward dosage adjustment of BeneFIX to as much as two times (2X) the initial empirically calculated dose in order to achieve the intended rise in circulating factor IX activity.
- •
- Monitor patients using a factor IX activity assay to ensure that the desired factor IX activity level has been achieved. Titrate the dose using the factor IX activity, pharmacokinetic parameters, such as half-life and recovery, as well as taking the clinical situation into consideration in order to adjust the dose as appropriate.
2.1 Dose
Calculating Initial Dose
Use the following formula to calculate the initial dose of BeneFIX:
number of factor IX IU required (IU)
=
body weight (kg)
×
desired factor IX increase (% of normal or IU/dL)
×
reciprocal of observed recovery (IU/kg per IU/dL)
Average Recovery
Adolescents/Adults (≥12 years)
In adults, on average, one International Unit (IU) of BeneFIX per kilogram of body weight increased the circulating activity of factor IX by 0.8 ± 0.2 IU/dL (range 0.4 to 1.2 IU/dL). Use the following formula to estimate the dose with 0.8 IU/dL average increase of factor IX per IU/kg body weight administered:
number of factor IX IU required (IU)
=
body weight (kg)
×
desired factor IX increase (% of normal or IU/dL)
×
1.3 (IU/kg per IU/dL)
Children (<12 years)
In children, on average, one international unit of BeneFIX per kilogram of body weight increased the circulating activity of factor IX by 0.7 ± 0.3 IU/dL (range 0.2 to 2.1 IU/dL; median of 0.6 IU/dL per IU/kg). Use the following formula to estimate the dose with 0.7 IU/dL average increase of factor IX per IU/kg body weight administered:
number of factor IX IU required (IU)
=
body weight (kg)
×
desired factor IX increase (% of normal or IU/dL)
×
1.4 (IU/kg per IU/dL)
Doses administered should be titrated to the patient's clinical response. Patients may vary in their pharmacokinetic (e.g., half-life, recovery) and clinical responses to BeneFIX. Although the dose can be estimated by the calculations above, it is highly recommended that, whenever possible, appropriate laboratory tests, including serial factor IX activity assays, be performed.
Dosing for On-demand Treatment and Control of Bleeding Episodes and Perioperative Management
Table: 1 Type of Hemorrhage
Circulating Factor IX Activity Required [% of normal or (IU/dL)]
Dosing Interval [hours]
Duration of Therapy [days]
Minor
Uncomplicated hemarthroses, superficial muscle, or soft tissue
20-30
12-24
1-2
Moderate
Intramuscle or soft tissue with dissection, mucous membranes, dental extractions, or hematuria
25-50
12-24
Treat until bleeding stops and healing begins, about 2 to 7 days
Major
Pharynx, retropharynx, retroperitoneum, CNS, surgery
50-100
12-24
7-10
Adapted from: Roberts and Eberst1.
Routine Prophylaxis
For long-term prophylaxis against bleeding, the recommended regimen is 100 IU/kg once weekly. Children (<12 years) have lower recovery, shorter half-life and higher clearance (based on per kg body weight) as compared to adolescents and adults. Adjust the dosing regimen (dose or frequency) based on the patient's clinical response.
2.2 Preparation and Reconstitution
The procedures below are provided as general guidelines for the preparation and reconstitution of BeneFIX.
Preparation
- 1.
- Always wash hands before performing the following procedures.
- 2.
- Use aseptic technique (meaning clean and germ-free) during the reconstitution procedure.
- 3.
- Use all components in the reconstitution and administration of this product as soon as possible after opening their sterile containers to minimize unnecessary exposure to the atmosphere.
- 4.
- Pooling: If needing more than one vial of BeneFIX per infusion, reconstitute each vial according to the following instructions. Remove the diluent syringe leaving the vial adapter in place, and use a separate large luer lock syringe to draw back the reconstituted contents of each vial. Do not detach the diluent syringes or the large luer lock syringe until you are ready to attach the large luer lock syringe to the next vial adapter.
Reconstitution
- 1.
- If refrigerated, allow the vial of lyophilized BeneFIX and the pre-filled diluent syringe to reach room temperature.
- 2.
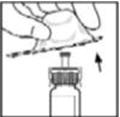
- Remove the plastic flip-top cap from the BeneFIX vial to expose the central portions of the rubber stopper.
- 3.
- Wipe the top of the vial with the alcohol swab provided, or use another antiseptic solution, and allow to dry. After cleaning, do not touch the rubber stopper with your hand or allow it to touch any surface.
- 4.
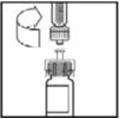
- Peel back the cover from the clear plastic vial adapter package. Do not remove the adapter from the package.
- 5.
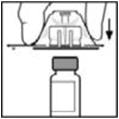
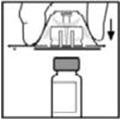
- Place the vial on a flat surface. While holding the adapter in the package, place the vial adapter over the vial and press down firmly on the package until the adapter spike penetrates the vial stopper.
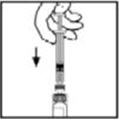
- 6.
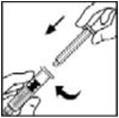
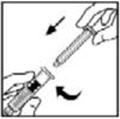
- Grasp the plunger rod as shown in the diagram. Avoid contact with the shaft of the plunger rod. Attach the threaded end of the plunger rod to the diluent syringe plunger by pushing and turning firmly.
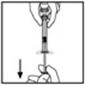
- 7.
- Break off the tamper-resistant plastic-tip cap from the diluent syringe by snapping the perforation of the cap. Do not touch the inside of the cap or the syringe tip. The diluent syringe may need to be recapped (if not administering reconstituted BeneFIX immediately), so place the cap on its top on a clean surface in a spot where it would be least likely to become environmentally contaminated.
- 8.
- Lift the package away from the adapter and discard the package.
- 9.
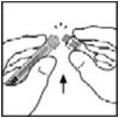
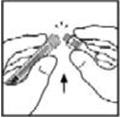
- Place the vial on a flat surface. Connect the diluent syringe to the vial adapter by inserting the tip into the adapter opening while firmly pushing and turning the syringe clockwise until secured.
- 10.
- Slowly depress the plunger rod to inject all the diluent into the BeneFIX vial.
- 11.
- Without removing the syringe, gently swirl the contents of the vial until the powder is dissolved.
- 12.
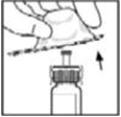
- Invert the vial and slowly draw the solution into the syringe.
- 13.
- Detach the syringe from the vial adapter by gently pulling and turning the syringe counter-clockwise. Discard the vial with the adapter attached.
- 14.
- The reconstituted solution should be clear and colorless. If it is not, discard and use a new kit. If the solution is not to be used immediately, recap the syringe. Do not touch the syringe tip or the inside of the cap.
- 15.
- Store the reconstituted solution at room temperature and use it within 3 hours.
Note: BeneFIX, when reconstituted, contains polysorbate-80, which is known to increase the rate of di-(2-ethylhexyl) phthalate (DEHP) extraction from polyvinyl chloride (PVC). This should be considered during the preparation and administration of BeneFIX, including storage time elapsed in a PVC container following reconstitution. It is important that the recommendations for dosage and administration be followed closely [see Dosage and Administration (2.1, 2.3)].
Note: The tubing of the infusion set included with this kit does not contain DEHP.
2.3 Administration
For intravenous use after reconstitution only.
The safety and efficacy of administration by continuous infusion have not been established [see Warnings and Precautions (5.2)].
- •
- Inspect BeneFIX solution for particulate matter and discoloration prior to administration, whenever solution and container permit.
- •
- Administer BeneFIX using the tubing provided in this kit, and the pre-filled diluent syringe provided, or a single sterile disposable plastic syringe.
- •
- Do not mix or administer BeneFIX in the same tubing or container with other medicinal products.
Administration
- 1.
- Attach the syringe to the luer end of the infusion set tubing provided.
- 2.
- Apply a tourniquet and prepare the injections site by wiping the skin well with an alcohol swab provided in the kit.
- 3.
- Perform venipuncture. Insert the needle on the infusion set tubing into the vein, and remove the tourniquet. Inject the reconstituted BeneFIX intravenously over several minutes. Adjust the rate of administration based on the patient's comfort level.
Note: Agglutination of red blood cells in the tubing/syringe has been reported with the administration of BeneFIX. No adverse events have been reported in association with this observation. To minimize the possibility of agglutination, it is important to limit the amount of blood entering the tubing. Blood should not enter the syringe. If red blood cell agglutination is observed in the tubing or syringe, discard all material (tubing, syringe and BeneFIX solution) and resume administration with a new package. - 4.
- Following completion of BeneFIX treatment, remove and discard the infusion set. Dispose of all unused solution, empty vial(s), and used needles and syringes in an appropriate container.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis, have been reported with BeneFIX and have manifested as pruritus, rash, urticaria, hives, facial swelling, dizziness, hypotension, nausea, chest discomfort, cough, dyspnea, wheezing, flushing, discomfort (generalized) and fatigue. Frequently, these events have occurred in close temporal association with the development of factor IX inhibitors.
Closely monitor patients for signs and symptoms of acute hypersensitivity reactions, particularly during the early phases of initial exposure to product. Because of the potential for allergic reactions with factor IX concentrates, perform the initial (approximately 10 – 20) administrations of factor IX under medical supervision where proper medical care for allergic reactions could be provided. Advise patients to discontinue use of the product and contact their physician and/or seek immediate emergency care. Immediately discontinue the administration and initiate appropriate treatment if symptoms occur.
BeneFIX contains trace amounts of hamster (CHO) proteins. Patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
5.2 Thromboembolic Complications
There have been post-marketing reports of thrombotic events in patients receiving continuous-infusion BeneFIX through a central venous catheter, including life-threatening superior vena cava (SVC) syndrome in critically ill neonates [see Adverse Reactions (6.2)]. The safety and efficacy of BeneFIX administration by continuous infusion have not been established [see Dosage and Administration (2.1, 2.3)].
5.3 Nephrotic Syndrome
Nephrotic syndrome has been reported following immune tolerance induction with factor IX products in hemophilia B patients with factor IX inhibitors and a history of allergic reactions to factor IX. The safety and efficacy of using BeneFIX for immune tolerance induction have not been established.
5.4 Neutralizing Antibodies (Inhibitors)
Neutralizing antibodies (inhibitors) have been reported following administration of BeneFIX [see Adverse Reactions (6.1)]. Evaluate patients using BeneFIX for the development of factor IX inhibitors by appropriate clinical observations and laboratory tests. If expected plasma factor IX activity levels are not attained, or if bleeding is not controlled with an expected dose, perform an assay that measures factor IX inhibitor concentration.
Patients with factor IX inhibitors are at an increased risk of severe hypersensitivity reactions or anaphylaxis upon subsequent challenge with factor IX.2 Evaluate patients experiencing allergic reactions for the presence of an inhibitor and closely monitor patients with inhibitors for signs and symptoms of acute hypersensitivity reactions, particularly during the early phases of initial exposure to product [see Warnings and Precautions (5.1)].
5.5 Monitoring Laboratory Tests
- •
- Monitor patients for factor IX activity levels by the one-stage clotting assay to confirm that adequate factor IX levels have been achieved and maintained, when clinically indicated [see Dosage and Administration (2.1)].
- •
- Monitor patients for the development of inhibitors if expected plasma factor IX activity levels are not attained, or if bleeding is not controlled with the recommended dose of BeneFIX. Determine plasma factor IX inhibitor levels in Bethesda Units (BUs).
-
6 ADVERSE REACTIONS
The most serious adverse reactions are systemic hypersensitivity reactions, including bronchospastic reactions and/or hypotension and anaphylaxis and the development of high-titer inhibitors necessitating alternative treatments to factor IX replacement therapy.
The most common adverse reactions observed in clinical trials [frequency >5% of previously treated patients (PTPs) or previously untreated patients (PUPs)] were fever, cough, headaches, dizziness, nausea, injections site reaction, injection site pain and skin-related hypersensitivity reactions (e.g., rash, hives).
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
During uncontrolled, open-label clinical trials with BeneFIX conducted in PTPs, 113 adverse reactions with known or unknown relation to BeneFIX therapy were reported among 38.5% (25 of 65) of subjects (with some subjects reporting more than one event) who received a total of 7,573 infusions. These adverse reactions are summarized in Table 2.
Table 2: Adverse Reactions Reported for PTPs* - *
- Adverse reactions reported within 72 hours of an infusion of BeneFIX.
- †
- Low-titer transient inhibitor formation.
- ‡
- The renal infarct developed in a hepatitis C antibody-positive patient 12 days after a dose of BeneFIX for a bleeding episode. The relationship of the infarct to the prior administration of BeneFIX is uncertain.
Body System
Adverse Reaction
Number of Patients (%)
Blood and lymphatic system disorders
Factor IX inhibition†
1 (1.5%)
Eye disorders
Blurred vision
1 (1.5%)
Gastrointestinal disorders
Nausea
4 (6.2%)
Vomiting
1 (1.5%)
General disorders and administration site conditions
Injection site reaction
5 (7.7%)
Injection site pain
4 (6.2%)
Fever
2 (3.1%)
Infections and infestations
Cellulitis at IV site
1 (1.5%)
Phlebitis at IV site
1 (1.5%)
Nervous system disorders
Headache
7 (10.8%)
Dizziness
5 (7.7%)
Taste perversion (altered taste)
3 (4.6%)
Shaking
1 (1.5%)
Drowsiness
1 (1.5%)
Renal and urinary disorders
Renal infarct‡
1 (1.5%)
Respiratory, thoracic and mediastinal disorders
Dry cough
1 (1.5%)
Hypoxia
1 (1.5%)
Chest tightness
1 (1.5%)
Skin and subcutaneous disorders
Rash
4 (6.2%)
Hives
2 (3.1%)
Vascular disorders
Flushing
2 (3.1%)
In the 63 PUPs, who received a total of 5,538 infusions, 10 adverse reactions were reported among 9.5% of the patients (6 out of 63) having known or unknown relationship to BeneFIX. These events are summarized in Table 3.
Table 3: Adverse Reactions Reported for PUPs* Body System
Adverse Reaction
Number of Patients (%)
Blood and lymphatic system disorders
Factor IX inhibition†
2 (3.2%)
General disorders and administration site conditions
Injection site reaction
1 (1.6%)
Chills
1 (1.6%)
Respiratory, thoracic and mediastinal disorders
Dyspnea (respiratory distress)
2 (3.2%)
Skin and subcutaneous disorders
Hives
3 (4.8%)
Rash
1 (1.6%)
In a multi-center, prospective, open-label clinical trial with BeneFIX administered at 100 IU/kg once weekly, 25 PTPs (exposed to a factor IX containing product for ≥100 exposure days) were evaluated, with 25 subjects treated for approximately 52 weeks. Common (≥5%) adverse reactions were headache (36%), fever (20%), and cough (8%). No subject was withdrawn from the trial due to an adverse reaction. In the trial, no inhibitors were detected and no thrombotic events or anaphylactic reactions were reported.
Immunogenicity
In clinical trials with 65 PTPs (defined as having more than 50 exposure days), a low-titer inhibitor was observed in one patient. The inhibitor was transient, the patient continued on the trial and had normal factor IX recovery at the trial completion (approximately 15 months after inhibitor detection).
In clinical trials with pediatric PUPs, inhibitor development was observed in 2 out of 63 patients (3.2%), both were high-titer (>5 BU) inhibitors detected after 7 and 15 exposure days, respectively. Both patients were withdrawn from the trial.
In a clinical trial of 25 PTPs, with BeneFIX administered at 100 IU/kg once weekly, no inhibitors were detected.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to BeneFIX with the incidence of antibodies to other products may be misleading.
Thromboembolic complications
All subjects participating in the PTP, PUP and surgery trials were monitored for clinical evidence of thrombosis. No thrombotic complications were reported in PUPs or surgery subjects. One PTP subject experienced a renal infarct (see Table 2). Laboratory studies of thrombogenicity (fibrinopeptide A and prothrombin fragment 1 + 2) were obtained in 41 PTPs and 7 surgery subjects prior to infusion and up to 24 hours following infusion. The results of these studies were inconclusive. Out of 29 PTP subjects noted to have elevated fibrinopeptide A levels post-infusion of BeneFIX, 22 also had elevated levels at baseline. Surgery subjects showed no evidence of significant increase in coagulation activation.
6.2 Postmarketing Experience
The following post-marketing adverse reactions have been reported for BeneFIX: inadequate factor IX recovery, inadequate therapeutic response, inhibitor development [see Clinical Pharmacology (12)], anaphylaxis [see Warnings and Precautions (5.1)], angioedema, dyspnea, hypotension, and thrombosis.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
There have been post-marketing reports of thrombotic events, including life-threatening SVC syndrome in critically ill neonates, while receiving continuous-infusion BeneFIX through a central venous catheter. Cases of peripheral thrombophlebitis and DVT have also been reported. In some, BeneFIX was administered via continuous infusion, which is not an approved method of administration [see Dosage and Administration (2)]. The safety and efficacy of BeneFIX administration by continuous infusion have not been established [see Warnings and Precautions (5.2)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with BeneFIX use in pregnant women to inform a drug-associated risk. Animal reproduction studies have not been conducted with BeneFIX. It is not known whether BeneFIX can affect reproductive capacity or cause fetal harm when given to pregnant women.
In the U.S. general population, the estimated background risk of major birth defect and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of BeneFIX in human milk, the effect on the breastfed infant, and the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for BeneFIX and any potential adverse effects on the breastfed child from BeneFIX or from the underlying maternal condition.
8.4 Pediatric Use
Safety, efficacy, and pharmacokinetics of BeneFIX have been evaluated in previously treated (PTP) and previously untreated pediatric patients (PUP) [see Clinical Studies (14) and Adverse Reactions (6)]. On average, lower recovery, shorter half-life and higher clearance (based on kg body weight) have been observed in children younger than 12 years old [see Clinical Pharmacology (12.3)]. Dose adjustment may be needed [see Dosage and Administration (2.1)].
8.5 Geriatric Use
Clinical trials of BeneFIX did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Dose selection for an elderly patient should be individualized [see Dosage and Administration (2.1)].
-
11 DESCRIPTION
BeneFIX, Coagulation Factor IX (Recombinant), is a purified protein produced by recombinant DNA technology. The product is formulated as a sterile, non-pyrogenic, lyophilized powder preparation intended to be reconstituted for intravenous injection. It is available in single-use vials containing the labeled amount of factor IX activity, expressed in International Units (IU). Each vial contains nominally 250, 500, 1000, 2000, or 3000 IU of recombinant coagulation factor IX. The potency (in IU) is determined using an in vitro one-stage clotting assay against the World Health Organization (WHO) International Standard for Factor IX concentrate. One IU is the amount of factor IX activity present in 1 mL of pooled, normal human plasma. After reconstitution of the lyophilized drug product, the concentrations of excipients are 0.234% sodium chloride, 8 mM L-histidine, 0.8% sucrose, 208 mM glycine, 0.004% polysorbate 80. The specific activity of BeneFIX is greater than or equal to 200 IU per milligram of protein. BeneFIX contains no preservatives and all dosage strengths yield a clear, colorless solution upon reconstitution.
Coagulation factor IX is the active ingredient in BeneFIX. It has a primary amino acid sequence that is identical to the Ala148 allelic form of human factor IX, and has structural and functional characteristics similar to those of endogenous factor IX.
BeneFIX is not derived from human blood. It is produced by a genetically engineered Chinese hamster ovary (CHO) cell line that is extensively characterized. No additives of animal or human origin are used during the cell culture, purification, and formulation processes of BeneFIX. The stored cell banks are free of human blood or plasma products. The CHO cell line secretes recombinant factor IX into a defined cell culture medium, and the recombinant factor IX is purified by a four-step chromatography purification process that does not require a monoclonal antibody step. The process also includes a membrane nanofiltration step that has the ability to retain molecules with apparent molecular weights >70,000 Da (such as large proteins and viral particles). BeneFIX is a single component by SDS-polyacrylamide gel electrophoresis evaluation.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
BeneFIX temporarily replaces the missing clotting factor IX that is needed for effective hemostasis.
12.2 Pharmacodynamics
The activated partial thromboplastin time (aPTT) is prolonged in people with hemophilia B. Treatment with factor IX concentrate may normalize the aPTT by temporarily replacing the factor IX. The administration of BeneFIX increases plasma levels of factor IX, and can temporarily correct the coagulation defect in these patients.
12.3 Pharmacokinetics
In a randomized, cross-over pharmacokinetic study, BeneFIX reconstituted in 0.234% sodium chloride diluent was shown to be pharmacokinetically equivalent to the previously marketed BeneFIX (reconstituted with Sterile Water for Injection) in 24 previously treated patients (≥12 years) at a dose of 75 IU/kg. In addition, pharmacokinetic parameters were followed up in 23 previously treated patients after repeated administration of BeneFIX for six months and found to be unchanged compared with those obtained at the initial evaluation. A summary of pharmacokinetic data are presented in Table 4:
Table 4: Pharmacokinetic Parameter Estimates for BeneFIX (75 IU/kg) at Baseline and Month 6 in Previously Treated Patients with Hemophilia B Abbreviations: AUC∞ = area under the plasma concentration-time curve from time zero to infinity; Cmax = peak concentration; t1/2 = plasma elimination half-life; CL = clearance; SD = standard deviation. Parameter
Baseline
N = 24
Mean ± SDMonth 6
N = 23
Mean ± SDCmax (IU/dL)
54.5 ± 15.0
57.3 ± 13.2
AUC∞ (IU∙hr/dL)
940 ± 237
923 ± 205
t1/2 (hr)
22.4 ± 5.3
23.8 ± 6.5
CL (mL/hr/kg)
8.47 ± 2.12
8.54 ± 2.04
Recovery
(IU/dL per IU/kg)0.73 ± 0.20
0.76 ± 0.18
Pediatric Patients (<12 years)
A population pharmacokinetic model was developed using data collected in patients aged 7 months to 60 years who received single doses of BeneFIX ranging from 50 to 75 IU/kg. The parameters estimated using the final 2-compartment model are shown in Table 5. Infants and children had higher clearance, larger volume of distribution, shorter half-life and lower recovery than adolescents and adults.
Table 5: Mean ± SD Pharmacokinetic Parameters Derived from Population Pharmacokinetic Analysis Age Group Infants (<2 years) Children
2 to <6 yrChildren
6 to <12 yrAdolescents and Adults (≥12 years) Abbreviations: SD = standard deviation; Vss = volume of distribution at steady-state. Number of subjects
7
16
1
43
Clearance (mL/h/kg)
13.1 ± 2.1
13.1 ± 2.8
15.5
8.4 ± 2.4
Vss (mL/kg)
252 ± 35
257 ± 25
303
229 ± 57
Half-life (h)
15.6 ± 1.2
16.7 ± 1.9
16.3
23.1 ± 4.4
Recovery (IU/dL per IU/kg)
0.61 ± 0.10
0.60 ± 0.08
0.47
0.72 ± 0.19
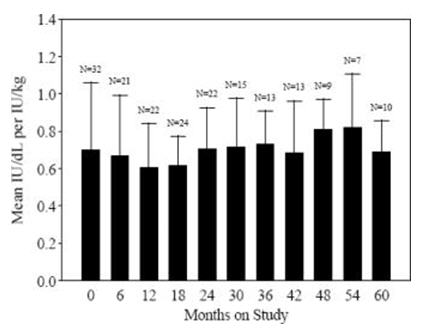
Data from 57 PUP subjects who underwent repeat recovery testing for up to 60 months demonstrated that the average recovery was consistent over time, as shown in Figure 1.
Figure 1. Average Recovery over Time
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
Efficacy of BeneFIX has been evaluated in clinical trials in which a total of 153 subjects received BeneFIX either for the on-demand treatment and control of bleeding episodes, perioperative management, and routine prophylaxis in patients with hemophilia B.
On-demand Treatment and Control of Bleeding Episodes
Fifty-six PTPs and sixty-three PUPs were treated for bleeding episodes on an on-demand treatment and control of bleeds (see Tables 6 and 7). The PTPs were followed over a median interval of 24 months (mean 23.4 ± 5.3 months) and for a median of 83.5. The PUPs were followed over a median interval of 37 months (mean 38.1 ± 16.4 months) and for a median of 89 exposure days.
Fifty-five PTPs and fifty-four PUPs received BeneFIX for the treatment of bleeding episodes (see Table 6). Bleeding episodes that were managed successfully included hemarthrosis and bleeding in soft tissue and muscle. Data concerning the severity of bleeding episodes were not reported. In the PTPs, 88% of total infusions administrated for on-demand treatment were rated as an "excellent" or "good" response.
Table 6: Efficacy of BeneFIX for On-Demand Treatment in PTPs and PUPs - *
- Response ratings not provided for 1.3% and 2% of 1st infusions for PTPs and PUPs, respectively.
- †
- One subject discontinued the trial after one month of treatment due to bleeding episodes that were difficult to control; he did not have a detectable inhibitor.
- ‡
- Three subjects were not successfully treated including one episode in a subject due to delayed time to infusion and insufficient dosing and in 2 subjects due to inhibitor formation.
Median Dose: IU/kg (range)
Rate of Bleeds Resolved with 1 Infusion
Response to 1st Infusion Rating*
Excellent/Good
Response to 1st Infusion Rating*
Moderate
Response to 1st Infusion Rating*
No Response
PTPs
N = 55†
42.8
(6.5–224.6)81%
90.9%
7.1%
0.7%
PUPs
N = 54‡
62.7
(8.2–292)75%
94.1%
2.9%
1.0%
A total of 20 PTPs were treated with BeneFIX for secondary prophylaxis (the regular administration of FIX replacement therapy to prevent bleeding in patients who may have already demonstrated clinical evidence of hemophilic arthropathy or joint disease) at some regular interval during the trial with a mean of 2 infusions per week (see Table 7). Thirty-two PUPs were administered BeneFIX for routine (primary and secondary) prophylaxis (see Table 7). Twenty-four PUPs were administered BeneFIX at least twice weekly, and eight PUPs were administered BeneFIX once weekly. Seven PTPs experienced a total of 26 spontaneous bleeding episodes within 48 hours after an infusion. Six spontaneous bleeds within 48 hours after an infusion were reported in 5 PUPs. Prophylaxis therapy was rated as "excellent" or "effective" in 93% of PTPs receiving prophylaxis one to two times per week.
Table 7: Efficacy of Prophylaxis of BeneFIX in PTPs and PUPs Total Exposure (infusions) Duration of Prophylaxis (months)
(mean ± SD)Dose IU/kg
(mean ± SD)Spontaneous Bleeds Within 48 hrs of Infusion Response Rating*
ExcellentResponse Rating*
EffectiveResponse Rating*
InadequateAbbreviation: SD = standard deviation. 20 PTPs
2985
18.2 ± 8.4†
40.3 ± 15.2†
28
56.0%
37.1%
4.3%
32 PUPs
3158
14.4 ± 8.1
73.3 ± 33.1
6
91.3%
6.4%
1.7%
Perioperative Management
Management of hemostasis was evaluated in the surgical setting in both PTPs and PUPs (see Table 8 and Table 9). Thirty-six surgical procedures have been performed in 28 PTPs with 23 major surgical procedures performed (including 6 complicated dental extractions). Thirty surgical procedures have been performed in 23 PUPs. Twenty-eight of these procedures were considered minor. Hemostasis was maintained throughout the surgical period; however, one PTP subject required evacuation of a surgical wound-site hematoma, and another PTP subject who received BeneFIX after a tooth extraction required further surgical intervention due to oozing at the extraction site. There was no clinical evidence of thrombotic complications in any of the subjects.
Among the PTP surgery subjects, the median increase in circulating factor IX activity was 0.7 IU/dL per IU/kg infused (range 0.3–1.2 IU/dL; mean 0.8 ± 0.2 IU/dL per IU/kg). The median elimination half-life for the PTP surgery subjects was 19.4 hours (range 10–37 hours; mean 21.3 ± 8.1 hours).
Table 8: Efficacy of BeneFIX for Surgical Procedures in Previously Treated Patients - *
- Some surgery types were done as a part of a single procedure.
- †
- Response assessment not provided for 1 procedure.
- ‡
- Includes pulse and continuous-infusion regimens; CI counted as 1 procedure in this summary.
- §
- Includes complicated extractions (6), clearance, and fillings.
- ¶
- E.g., punch biopsy of the skin.
Surgery Type*
Number of Procedures
(Number of Subjects)Response
Excellent/Good
Response
Moderate
Response
No Response
Ankle surgery
2 (2)
2 (100%)
-
-
Hip prosthesis implant (right)
1 (1)
1 (100%)
-
-
Knee arthroplasty (2 bilateral, 1 right)
3 (3)
3 (100%)
-
-
Knee arthroscopic synovectomy
2 (2)†
1 (50%)
-
-
Liver transplantation (orthotopic)
1 (1)
1 (100%)
-
-
Splenectomy
1 (1)
1 (100%)
-
-
External fixation device removal (wrist)
1 (1)
1 (100%)
-
-
Hernia repair
3 (2)
3 (100%)
-
-
Subacromial decompression (left)
1 (1)
1 (100%)
-
-
Calf debridement, dental extraction‡
1 (1)
1 (100%)
-
-
Lymph node removal, dental extraction‡
1 (1)
1 (100%)
-
-
Left heel cord lengthening
1 (1)
1 (100%)
-
-
Dental procedures§
12 (11)
11 (92%)
1 (8%)
-
Minor procedures¶
6 (6)
6 (100%)
-
-
Table 9: Efficacy of BeneFIX for Surgical Procedures in Previously Untreated Patients Surgery Type*
Number of Procedures (Number of Subjects)
Response
Excellent/Good
Response
Moderate
Response
No Response
Hernia repair
2 (2)
2 (100%)
-
-
Minor procedures†
28 (21)‡
27 (96%)
-
-
Nine of the major surgical procedures were performed in 8 PUPs using a continuous-infusion regimen. Five of the surgical procedures were performed in PUPs using a continuous-infusion regimen over 3 to 5 days. Although circulating factor IX levels targeted to restore and maintain hemostasis were achieved with both pulse replacement and continuous infusion regimens, clinical trial experience with continuous infusion of BeneFIX for perioperative management in hemophilia B has been too limited to establish the safety and clinical efficacy of administration of the product by continuous infusion.
Routine Prophylaxis
In an open-label trial of 25 patients (age range 12–54 years) comparing on-demand treatment versus prophylaxis when administered at a dose of 100 IU/kg once weekly, the annualized bleed rate (ABR) for the prophylaxis period was significantly lower (p < 0.0001) than the ABR for the on-demand period (mean ± standard deviation (SD): 3.6 ± 4.6, median: 2.0, min–max: 0–13.8 versus mean: 32.9 ± 17.4, median: 33.6, min–max: 6.1–69.0, respectively).
In an open-label crossover trial in patients aged 6–64 years, of 100 IU/kg once weekly (44 patients) and 50 IU/kg twice weekly (43 patients) with 4-month treatment periods, the ABR for the 100 IU/kg once-weekly prophylaxis period was mean 4.4 ± 10.0 episodes per year (median: 0.0, min–max: 0 – 50.5) and mean 2.8 ± 5.7 (median: 0.0, min–max: 0 – 24.1) for the 50 IU/kg twice-weekly period. Six patients aged <12 years had mean ABR of 1.6 ± 1.7 (median: 1.5, min–max: 0–3.3) in the 100 IU/kg once-weekly period, and mean ABR of 0 ± 0 (median: 0, min–max: 0–0) in the 50 IU/kg twice-weekly period.
-
15 REFERENCES
- 1.
- Roberts HR, Eberst ME. Current management of hemophilia B. Hematol Oncol Clin North Am. 1993;7(6):1269–1280.
- 2.
- Shapiro AD, Ragni MV, Lusher JM, et al. Safety and efficacy of monoclonal antibody purified factor IX concentrate in previously untreated patients with hemophilia B. Thromb Haemost. 1996;75(1):30–35.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
BeneFIX, Coagulation Factor IX (Recombinant), is supplied in kits that include single-use vials which contain nominally 250, 500, 1000, 2000, or 3000 IU per vial with sterile pre-filled diluent syringe, vial adapter reconstitution device, sterile infusion set, and two (2) alcohol swabs, one bandage, and one gauze pad. The drug product, diluents for injection and the rest of components included within the BeneFIX 250, 500, 1000, 2000 or 3000 IU kit are not made from natural rubber and natural rubber latex. Actual factor IX activity in IU is stated on the label of each vial.
BeneFIX
Nominal Strengths
Color Code
Kit NDC Number
250 International Units
Yellow
58394-633-03
500 International Units
Blue
58394-634-03
1000 International Units
Green
58394-635-03
2000 International Units
Red
58394-636-03
3000 International Units
Grey
58394-637-03
-
17 PATIENT COUNSELING INFORMATION
- •
- Advise patients to read the FDA-approved patient labeling (Patient Information and Instructions for Use)
- •
- Allergic-type hypersensitivity reactions are possible. Inform patients of the early signs of hypersensitivity reactions [including hives (rash with itching), generalized urticaria, tightness of the chest, wheezing, hypotension] and anaphylaxis. Advise patients to discontinue use of the product and contact their physicians if these symptoms occur.
- •
- Advise patients to contact their physician or treatment facility for further treatment and/or assessment if they experience a lack of a clinical response to factor IX replacement therapy, as in some cases this may be a manifestation of an inhibitor.
-
SPL UNCLASSIFIED SECTION
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
For Medical Information about BENEFIX, please visit www.pfizermedinfo.com or call 1-800-438-1985.
-
FDA-Approved Patient Labeling
Patient Information
BeneFIX® / BEN-uh-fiks/
[Coagulation Factor IX (Recombinant)]
Please read this Patient Leaflet carefully before using BeneFIX and each time you get a refill. There may be new information. This Patient Leaflet does not take the place of talking with your doctor about your medical condition or your treatment.
What is BeneFIX?
BeneFIX is an injectable medicine that is used to help control and prevent bleeding in people with hemophilia B. Hemophilia B is also called congenital factor IX deficiency or Christmas disease.
BeneFIX is NOT used to treat hemophilia A.
What should I tell my doctor before using BeneFIX?
Tell your doctor and pharmacist about all of the medicines you take, including all prescription and non-prescription medicines, such as over-the-counter medicines, supplements, or herbal medicines.
Tell your doctor about all of your medical conditions, including if you:
- •
- have any allergies, including allergies to hamsters.
- •
- are pregnant or planning to become pregnant. It is not known if BeneFIX may harm your unborn baby.
- •
- are breastfeeding. It is not known if BeneFIX passes into the milk and if it can harm your baby.
How should I infuse BeneFIX?
The initial administrations of BeneFIX should be administered under proper medical supervision, where proper medical care for severe allergic reactions could be provided.
See the step-by-step instructions for infusing BeneFIX at the end of this leaflet. You should always follow the specific instructions given by your doctor. The steps listed below are general guidelines for using BeneFIX. If you are unsure of the procedures, please call your doctor or pharmacist before using.
Call your doctor right away if bleeding is not controlled after using BeneFIX.
Your doctor will prescribe the dose that you should take.
Your doctor may need to test your blood from time to time.
BeneFIX should not be administered by continuous infusion.
What if I take too much BeneFIX?
Call your doctor if you take too much BeneFIX.
What are the possible side effects of BeneFIX?
Allergic reactions may occur with BeneFIX. Call your doctor or get emergency treatment right away if you have any of the following symptoms:
- wheezing
- difficulty breathing
- chest tightness
- turning blue (look at lips and gums)
- fast heartbeat
- swelling of the face
- faintness
- rash
- hives
Your body can also make antibodies, called "inhibitors," against BeneFIX, which may stop BeneFIX from working properly.
Some common side effects of BeneFIX are fever, cough, nausea, injection site reaction, injection site pain, headache, dizziness and rash.
BeneFIX may increase the risk of thromboembolism (abnormal blood clots) in your body if you have risk factors for developing blood clots, including an indwelling venous catheter through which BeneFIX is given by continuous infusion. There have been reports of severe blood clotting events, including life-threatening blood clots in critically ill neonates, while receiving continuous-infusion BeneFIX through a central venous catheter. The safety and efficacy of BeneFIX administration by continuous infusion have not been established.
These are not all the possible side effects of BeneFIX.
Tell your doctor about any side effect that bothers you or that does not go away.
How should I store BeneFIX?
DO NOT FREEZE BeneFIX kit.
BeneFIX kit can be stored at room temperature (below 86°F) or under refrigeration.
Throw away any unused BeneFIX and diluent after the expiration date indicated on the label.
Freezing should be avoided to prevent damage to the pre-filled diluent syringe.
BeneFIX does not contain a preservative. After reconstituting BeneFIX, you can store it at room temperature for up to 3 hours. If you have not used it in 3 hours, throw it away.
Do not use BeneFIX if the reconstituted solution is not clear and colorless.
What else should I know about BeneFIX?
Medicines are sometimes prescribed for purposes other than those listed here. Do not use BeneFIX for a condition for which it was not prescribed. Do not share BeneFIX with other people, even if they have the same symptoms that you have.
This Patient Leaflet summarizes the most important information about BeneFIX. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about BeneFIX that was written for healthcare professionals.
-
Instructions for Use
BeneFIX® / BEN-uh-fiks/
[Coagulation Factor IX (Recombinant)]
BeneFIX is supplied as a powder. Before it can be infused in your vein (intravenous injection), you must reconstitute the powder by mixing it with the liquid diluent supplied. The liquid diluent is 0.234% sodium chloride. BeneFIX should be reconstituted and infused using the infusion set, diluent, syringe, and adapter provided in this kit, and by following the directions below.
RECONSTITUTION
Always wash your hands before performing the following steps. Try to keep everything clean and germ-free while you are reconstituting BeneFIX. Once you open the vials, you should finish reconstituting BeneFIX as soon as possible. This will help keep the infusion set materials germ-free.
Note: If you use more than one vial of BeneFIX per infusion, reconstitute each vial according to steps 1 through 13.
- 1.
- If refrigerated, let the vial of BeneFIX and the pre-filled diluent syringe reach room temperature.
- 2.
- Remove the plastic flip-top cap from the BeneFIX vial to show the center part of the rubber stopper.
- 3.
- Wipe the top of the vial with the alcohol swab provided, or use another antiseptic solution, and allow to dry. After cleaning, do not touch the rubber stopper with your hand or allow it to touch any surface.
- 4.
- Peel back the cover from the clear plastic vial adapter package. Do not remove the adapter from the package.
- 5.
- Place the vial on a flat surface. While holding the adapter in the package, place the vial adapter over the vial. Press down firmly on the package until the adapter snaps into place on top of the vial, with the adapter spike penetrating the vial stopper.
- 6.
- Grasp the plunger rod as shown in the picture below. Do not touch the shaft of the plunger rod. Attach the threaded end of the plunger rod to the diluent syringe plunger by pushing and turning firmly.
- 7.
- Break off the tamper-resistant, plastic-tip cap from the diluent syringe by snapping the perforation of the cap. Do not touch the inside of the cap or the syringe tip. The diluent syringe may need to be recapped (if reconstituted BeneFIX is not used immediately), so place the cap on its tip on a clean surface in a spot where it will stay clean.
- 8.
- Lift the package away from the adapter and discard the package.
- 9.
- Place the vial on a flat surface. Connect the diluent syringe to the vial adapter by inserting the tip of the syringe into the adapter opening while firmly pushing and turning the syringe clockwise until the connection is secured.
- 10.
- Slowly push the plunger rod to inject all the diluent into the BeneFIX vial.
- 11.
- With the syringe still connected to the adapter, gently swirl the contents of the vial until the powder is dissolved.
Look at the final solution before infusing it. The solution should be clear to colorless. If it is not, throw away the solution and use a new kit. - 12.
- Make sure the syringe plunger rod is still fully pressed down, then turn over the vial. Slowly pull the solution into the syringe. Turn the syringe upward again and remove any air bubbles by gently tapping the syringe with your finger and slowly pushing air out of the syringe.
If you reconstituted more than one vial of BeneFIX, remove the diluent syringe from the vial adapter and leave the vial adapter attached to the vial. Quickly attach a separate large luer lock syringe and pull the reconstituted solution as instructed above. Repeat this procedure with each vial in turn. Do not detach the diluent syringes or the large luer lock syringe until you are ready to attach the large luer lock syringe to the next vial adapter.
- 13.
- Remove the syringe from the vial adapter by gently pulling and turning the syringe counter-clockwise. Throw away the vial with the adapter attached.
If you are not using the solution right away, you should carefully replace the syringe cap. Do not touch the syringe tip or the inside of the cap.
BeneFIX should be infused within 3 hours after reconstitution. The reconstituted solution may be stored at room temperature prior to infusion.
INFUSION (Intravenous Injection)
Continuous infusion is not an approved way to administer BeneFIX.
Your doctor or healthcare professional should teach you how to infuse BeneFIX. Once you learn how to self-infuse, you can follow the instructions in this insert.
- 1.
- Attach the syringe to the luer end of the provided infusion set tubing.
- 2.
- Apply a tourniquet and prepare the injection site by wiping the skin well with an alcohol swab provided in the kit.
- 3.
- Insert the butterfly needle of the infusion set tubing into your vein as instructed by your doctor or healthcare provider. Remove the tourniquet. Infuse the reconstituted BeneFIX product over several minutes. Your comfort level should determine the rate of infusion.
Clumping of red blood cells in the tubing/syringe has been reported with the administration of BeneFIX. No adverse events have been reported in association with this observation. To minimize the possibility of clumping it is important to limit the amount of blood entering the tubing. Blood should not enter the syringe.
Note: If red blood cell clumping is observed in the tubing or syringe, discard all material (tubing, syringe and BeneFIX solution) and continue administration with a new package. - 4.
- After infusing BeneFIX, remove the infusion set and discard. The amount of drug product left in the infusion set will not affect your treatment. Dispose of all unused solution, the empty vial(s), and the used needles and syringes in an appropriate container used for throwing away waste that might hurt others if not handled properly.
It is a good idea to record the lot number from the BeneFIX vial label every time you use BeneFIX. You can use the peel-off label found on the vial to record the lot number.
If you have any questions or concerns about BeneFIX, ask your doctor or healthcare provider.
US Govt. License No. 3
LAB-0464-15.0
-
PRINCIPAL DISPLAY PANEL - 5 mL Syringe Label – Used in All Kits
0.234% Sodium
Chloride Diluent
5 mLDisposable syringe for
drug diluent use with BeneFix®
Coagulation Factor IX (Recombinant)Sterile, nonpyrogenic. Contains no
preservatives.Manufactured for
Wyeth Pharmaceuticals LLC
A subsidiary of Pfizer Inc.
Philadelphia, PA 19101
Manufactured by
Vetter Pharma-Fertigung GmbH & Co. KG
Schuetzenstrasse 87, 99-101
88212 Ravensburg, Germany
or
Vetter Pharma-Fertigung GmbH & Co. KG
Eisenbahnstrasse 2-4
88085 Langenargen, Germany
US Govt. License No. 3Rx only
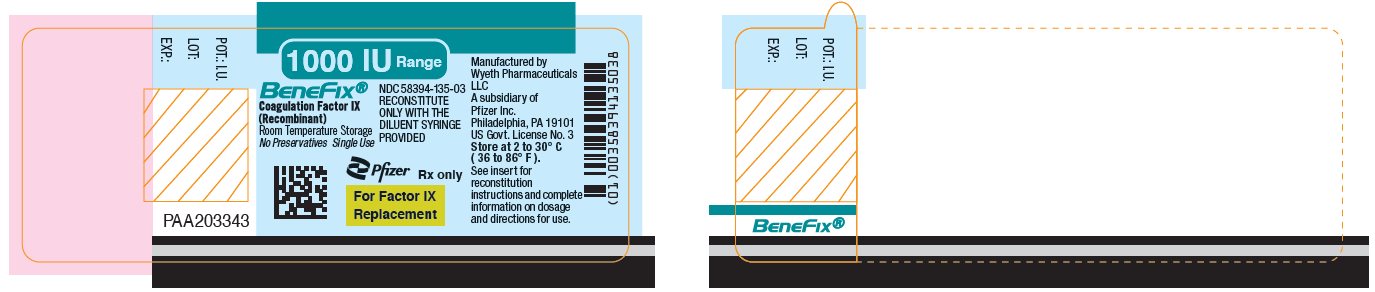
- PRINCIPAL DISPLAY PANEL - 1000 IU Vial Label
-
PRINCIPAL DISPLAY PANEL - 1000 IU Kit Carton
One single-use vial with 5-mL prefilled diluent syringe
NDC 58394-635-03Room Temperature Storage
1000 IU Range
BeneFix®
Coagulation Factor IX (Recombinant)
Room Temperature Storage
No Plasma. No Albumin.For Intravenous
AdministrationStore at 2 to 30º C (36 to 86º F).
For Factor IX Replacement Therapy Only
Reconstitute BeneFix® with the
enclosed sterile prefilled
syringe of 0.234% Sodium
Chloride for Injection, ONLY.Rx only
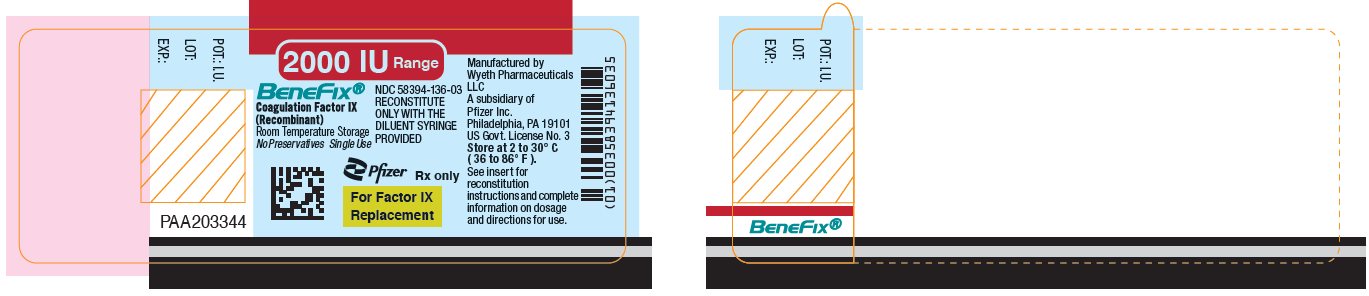
Pfizer - PRINCIPAL DISPLAY PANEL - 2000 IU Vial Label
-
PRINCIPAL DISPLAY PANEL - 2000 IU Kit Carton
One single-use vial with 5-mL prefilled diluent syringe
NDC 58394-636-03Room Temperature Storage
2000 IU Range
BeneFix®
Coagulation Factor IX (Recombinant)
Room Temperature Storage
No Plasma. No Albumin.For Intravenous
AdministrationStore at 2 to 30º C (36 to 86º F).
For Factor IX Replacement Therapy Only
Reconstitute BeneFix® with the
enclosed sterile prefilled
syringe of 0.234% Sodium
Chloride for Injection, ONLY.Rx only
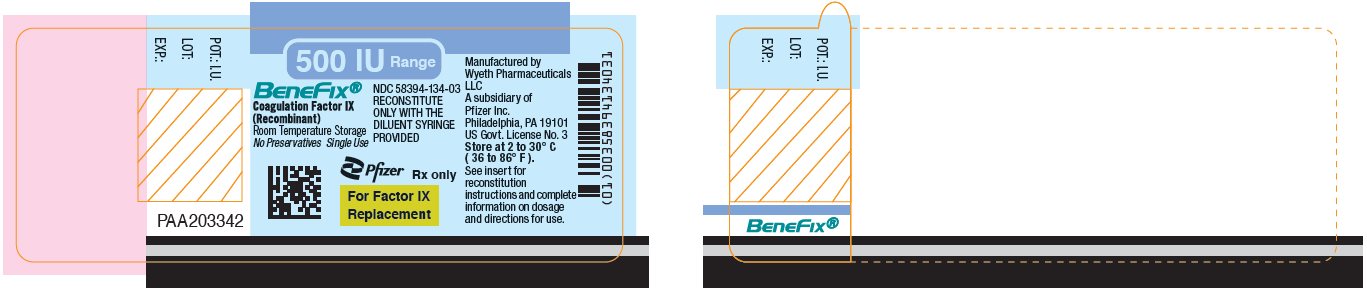
Pfizer - PRINCIPAL DISPLAY PANEL - 500 IU Vial Label
-
PRINCIPAL DISPLAY PANEL - 500 IU Kit Carton
One single-use vial with 5-mL prefilled diluent syringe
NDC 58394-634-03Room Temperature Storage
500 IU Range
BeneFix®
Coagulation Factor IX (Recombinant)
Room Temperature Storage
No Plasma. No Albumin.For Intravenous
AdministrationStore at 2 to 30º C (36 to 86º F).
For Factor IX Replacement Therapy Only
Reconstitute BeneFix® with the
enclosed sterile prefilled
syringe of 0.234% Sodium
Chloride for Injection, ONLY.Rx only
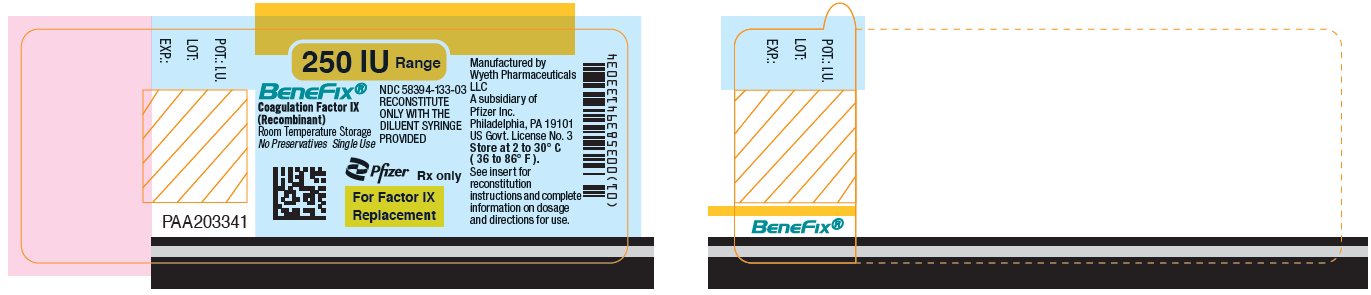
Pfizer - PRINCIPAL DISPLAY PANEL - 250 IU Vial Label
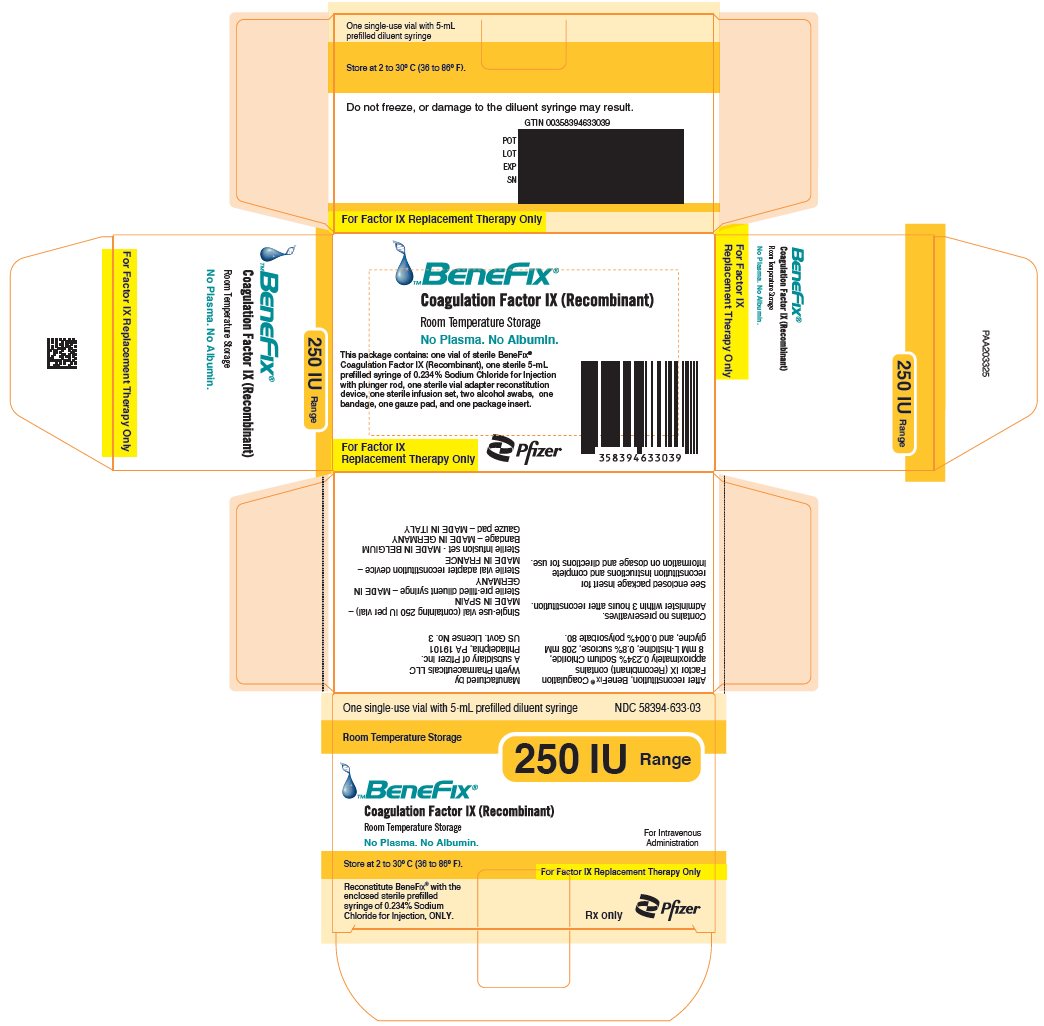
-
PRINCIPAL DISPLAY PANEL - 250 IU Kit Carton
One single-use vial with 5-mL prefilled diluent syringe
NDC 58394-633-03Room Temperature Storage
250 IU Range
BeneFix®
Coagulation Factor IX (Recombinant)
Room Temperature Storage
No Plasma. No Albumin.For Intravenous
AdministrationStore at 2 to 30° C (36 to 86° F).
For Factor IX Replacement Therapy Only
Reconstitute BeneFIX® with the
enclosed sterile prefilled
syringe of 0.234% Sodium
Chloride for Injection, ONLY.Rx only
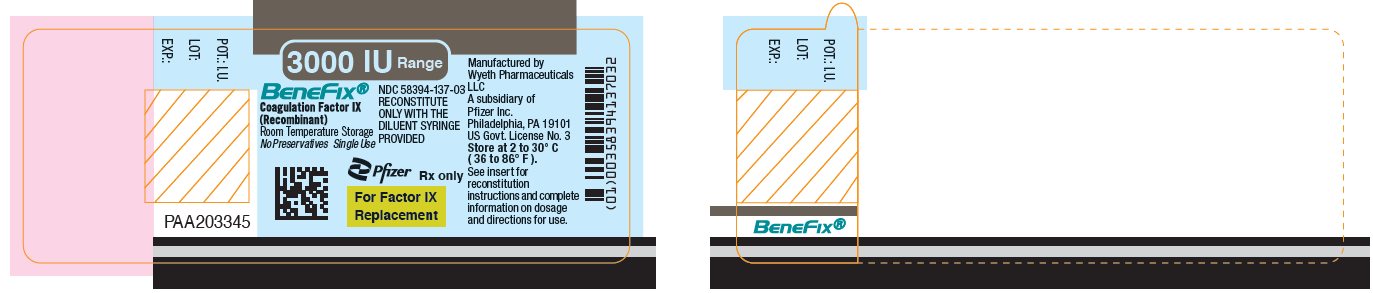
Pfizer - PRINCIPAL DISPLAY PANEL - 3000 IU Vial Label
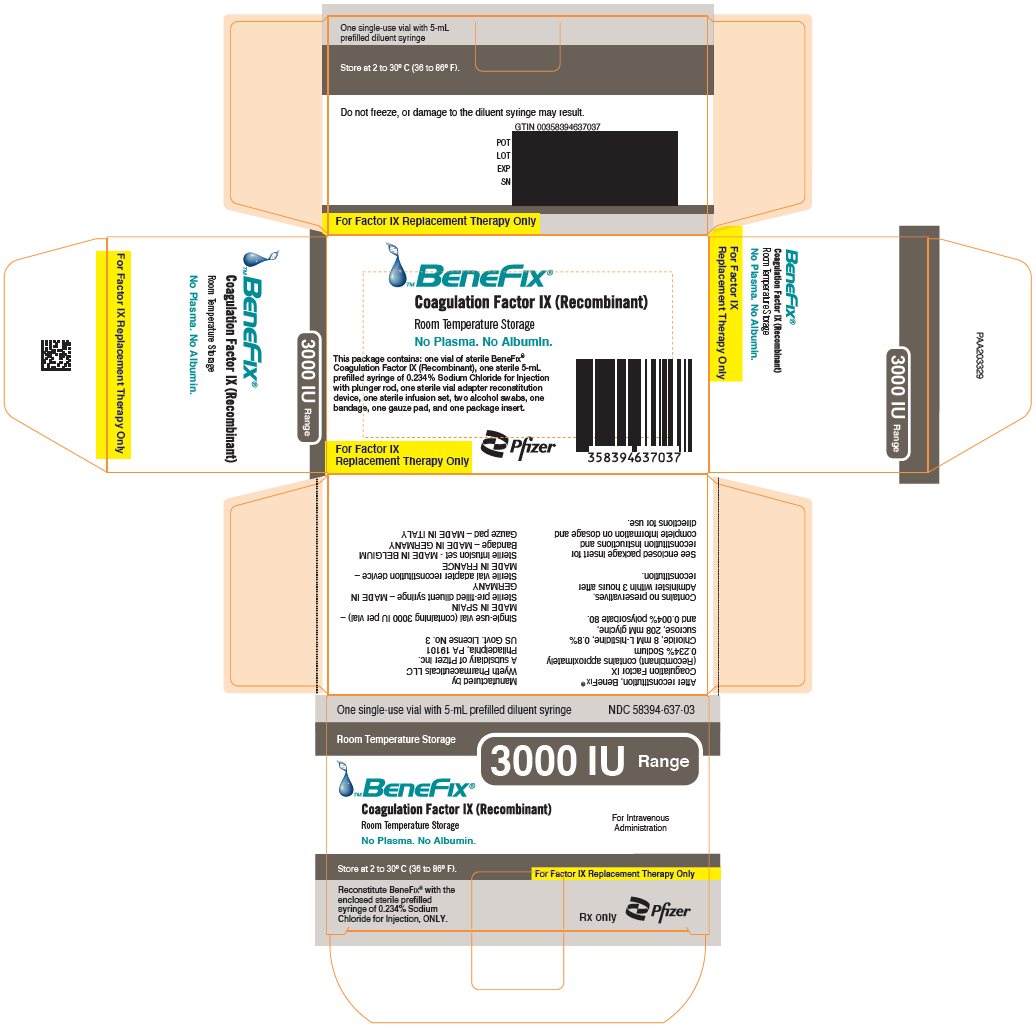
-
PRINCIPAL DISPLAY PANEL - 3000 IU Kit Carton
One single-use vial with 5-mL prefilled diluent syringe
NDC 58394-637-03Room Temperature Storage
3000 IU Range
BeneFix®
Coagulation Factor IX (Recombinant)
Room Temperature Storage
No Plasma. No Albumin.For Intravenous
AdministrationStore at 2 to 30º C (36 to 86º F).
For Factor IX Replacement Therapy Only
Reconstitute BeneFix® with the
enclosed sterile prefilled
syringe of 0.234% Sodium
Chloride for Injection, ONLY.Rx only
Pfizer -
INGREDIENTS AND APPEARANCE
BENEFIX
coagulation factor ix (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:58394-635 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58394-635-03 1 in 1 CARTON; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 5 mL Part 2 1 SYRINGE 5 mL Part 3 2 PACKET 2 mL Part 1 of 3 BENEFIX
coagulation factor ix (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:58394-135 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COAGULATION FACTOR IX RECOMBINANT HUMAN (UNII: 382L14738L) (COAGULATION FACTOR IX RECOMBINANT HUMAN - UNII:382L14738L) COAGULATION FACTOR IX RECOMBINANT HUMAN 1000 [iU] in 5 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) 6.2 mg in 5 mL GLYCINE (UNII: TE7660XO1C) 78.1 mg in 5 mL SUCROSE (UNII: C151H8M554) 40 mg in 5 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.22 mg in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58394-135-03 5 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103677 02/01/1997 Part 2 of 3 DILUENT
diluent injection, solutionProduct Information Item Code (Source) NDC:58394-041 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 11.93 mg in 5 mL WATER (UNII: 059QF0KO0R) 5 mL in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58394-041-11 5 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103677 02/01/1997 Part 3 of 3 ALCOHOL
alcohol swabProduct Information Item Code (Source) NDC:0069-1111 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-1111-01 1 mL in 1 PACKET; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103677 02/01/1997 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103677 02/01/1997 BENEFIX
coagulation factor ix (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:58394-636 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58394-636-03 1 in 1 CARTON; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 5 mL Part 2 1 SYRINGE 5 mL Part 3 2 PACKET 2 mL Part 1 of 3 BENEFIX
coagulation factor ix (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:58394-136 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COAGULATION FACTOR IX RECOMBINANT HUMAN (UNII: 382L14738L) (COAGULATION FACTOR IX RECOMBINANT HUMAN - UNII:382L14738L) COAGULATION FACTOR IX RECOMBINANT HUMAN 2000 [iU] in 5 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) 6.2 mg in 5 mL GLYCINE (UNII: TE7660XO1C) 78.1 mg in 5 mL SUCROSE (UNII: C151H8M554) 40 mg in 5 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.22 mg in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58394-136-03 5 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103677 02/01/1997 Part 2 of 3 DILUENT
diluent injection, solutionProduct Information Item Code (Source) NDC:58394-041 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 11.93 mg in 5 mL WATER (UNII: 059QF0KO0R) 5 mL in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58394-041-11 5 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103677 02/01/1997 Part 3 of 3 ALCOHOL
alcohol swabProduct Information Item Code (Source) NDC:0069-1111 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-1111-01 1 mL in 1 PACKET; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103677 02/01/1997 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103677 07/06/2007 BENEFIX
coagulation factor ix (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:58394-634 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58394-634-03 1 in 1 CARTON; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 5 mL Part 2 1 SYRINGE 5 mL Part 3 2 PACKET 2 mL Part 1 of 3 BENEFIX
coagulation factor ix (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:58394-134 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COAGULATION FACTOR IX RECOMBINANT HUMAN (UNII: 382L14738L) (COAGULATION FACTOR IX RECOMBINANT HUMAN - UNII:382L14738L) COAGULATION FACTOR IX RECOMBINANT HUMAN 500 [iU] in 5 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) 6.2 mg in 5 mL GLYCINE (UNII: TE7660XO1C) 78.1 mg in 5 mL SUCROSE (UNII: C151H8M554) 40 mg in 5 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.22 mg in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58394-134-03 5 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103677 02/01/1997 Part 2 of 3 DILUENT
diluent injection, solutionProduct Information Item Code (Source) NDC:58394-041 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 11.93 mg in 5 mL WATER (UNII: 059QF0KO0R) 5 mL in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58394-041-11 5 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103677 02/01/1997 Part 3 of 3 ALCOHOL
alcohol swabProduct Information Item Code (Source) NDC:0069-1111 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-1111-01 1 mL in 1 PACKET; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103677 02/01/1997 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103677 02/01/1997 BENEFIX
coagulation factor ix (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:58394-633 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58394-633-03 1 in 1 CARTON; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 5 mL Part 2 1 SYRINGE 5 mL Part 3 2 PACKET 2 mL Part 1 of 3 BENEFIX
coagulation factor ix (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:58394-133 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COAGULATION FACTOR IX RECOMBINANT HUMAN (UNII: 382L14738L) (COAGULATION FACTOR IX RECOMBINANT HUMAN - UNII:382L14738L) COAGULATION FACTOR IX RECOMBINANT HUMAN 250 [iU] in 5 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) 6.2 mg in 5 mL GLYCINE (UNII: TE7660XO1C) 78.1 mg in 5 mL SUCROSE (UNII: C151H8M554) 40 mg in 5 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.22 mg in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58394-133-03 5 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103677 02/01/1997 Part 2 of 3 DILUENT
diluent injection, solutionProduct Information Item Code (Source) NDC:58394-041 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 11.93 mg in 5 mL WATER (UNII: 059QF0KO0R) 5 mL in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58394-041-11 5 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103677 02/01/1997 Part 3 of 3 ALCOHOL
alcohol swabProduct Information Item Code (Source) NDC:0069-1111 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-1111-01 1 mL in 1 PACKET; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103677 02/01/1997 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103677 02/01/1997 BENEFIX
coagulation factor ix (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:58394-637 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58394-637-03 1 in 1 CARTON; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 5 mL Part 2 1 SYRINGE 5 mL Part 3 2 PACKET 2 mL Part 1 of 3 BENEFIX
coagulation factor ix (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:58394-137 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COAGULATION FACTOR IX RECOMBINANT HUMAN (UNII: 382L14738L) (COAGULATION FACTOR IX RECOMBINANT HUMAN - UNII:382L14738L) COAGULATION FACTOR IX RECOMBINANT HUMAN 3000 [iU] in 5 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) 6.2 mg in 5 mL GLYCINE (UNII: TE7660XO1C) 78.1 mg in 5 mL SUCROSE (UNII: C151H8M554) 40 mg in 5 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.22 mg in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58394-137-03 5 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103677 02/01/2012 Part 2 of 3 DILUENT
diluent injection, solutionProduct Information Item Code (Source) NDC:58394-041 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 11.93 mg in 5 mL WATER (UNII: 059QF0KO0R) 5 mL in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58394-041-11 5 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103677 02/01/1997 Part 3 of 3 ALCOHOL
alcohol swabProduct Information Item Code (Source) NDC:0069-1111 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-1111-01 1 mL in 1 PACKET; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103677 02/01/1997 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103677 02/01/2012 Labeler - Wyeth BioPharma Division of Wyeth Pharmaceuticals LLC (174350868) Establishment Name Address ID/FEI Business Operations Wyeth Farma SA 462005232 ANALYSIS(58394-633, 58394-634, 58394-635, 58394-636, 58394-637) , MANUFACTURE(58394-633, 58394-634, 58394-635, 58394-636, 58394-637) , PACK(58394-633, 58394-634, 58394-635, 58394-636, 58394-637) , LABEL(58394-633, 58394-634, 58394-635, 58394-636, 58394-637) Establishment Name Address ID/FEI Business Operations Vetter Pharma Fertigung GmbH & Co. KG (Ravensburg Schuetzenstrasse) 316126754 ANALYSIS(58394-633, 58394-634, 58394-635, 58394-636, 58394-637) , MANUFACTURE(58394-633, 58394-634, 58394-635, 58394-636, 58394-637) Establishment Name Address ID/FEI Business Operations Wyeth BioPharma Division of Wyeth Pharmaceuticals LLC 174350868 ANALYSIS(58394-633, 58394-634, 58394-635, 58394-636, 58394-637) , API MANUFACTURE(58394-633, 58394-634, 58394-635, 58394-636, 58394-637)