Label: REPAGLINIDE tablet
- NDC Code(s): 0574-0240-01, 0574-0241-01, 0574-0242-01
- Packager: Padagis US LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use REPAGLINIDE TABLETS safely and effectively. See full prescribing information for REPAGLINIDE TABLETS.
REPAGLINIDE tablets, for oral use
Initial U.S. Approval: 1997INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- •
- The recommended starting dose is 0.5 mg orally before each meal if HbA1c is less than 8%; and 1 or 2 mg orally before each meal if HbA1c is 8% or greater. (2.1)
- •
- The recommended dose range is 0.5 mg to 4 mg before meals, with a maximum daily dose of 16 mg. (2.1)
- •
- The patient’s dose should be doubled up to 4 mg with each meal until satisfactory glycemic control is achieved. At least one week should elapse to assess response after each dose adjustment. (2.1)
- •
- Instruct patients to skip the dose of repaglinide tablets if a meal is skipped. In patients who experience hypoglycemia, the dose of repaglinide tablets should be reduced. (2.1; 5.1)
- •
- Instruct patients to take repaglinide tablets within 30 minutes before meals. (2.1)
- •
- In patients with severe renal impairment (CrCl = 20 – 40 mL/min), recommended starting dose is 0.5 mg orally before each meal. (2.2)
- •
- Dose modifications are required when used concomitantly with some medications. (2.3,7)
DOSAGE FORMS AND STRENGTHS
Tablets: 0.5 mg, 1 mg, 2 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- •
- Hypoglycemia: Repaglinide tablets may cause hypoglycemia. Skip the scheduled dose of repaglinide tablets if a meal is skipped to reduce the risk of hypoglycemia. Reduce the dose of repaglinide tablets if hypoglycemia occurs. (5.1)
- •
- Serious Cardiovascular Adverse Reactions with Concomitant NPH-insulin: Repaglinide tablets are not indicated for use in combination with NPH-insulin. (5.2)
- •
- Macrovascular outcomes: There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with repaglinide tablets. (5.3)
ADVERSE REACTIONS
The most common adverse reactions (5% or greater incidence) among patients treated with repaglinide tablets were: hypoglycemia, upper respiratory infection, headache, sinusitis, arthralgia, nausea, diarrhea, and back pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Padagis at 1-866-634-9120 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- •
- Clopidogrel: Avoid concomitant use; if used concomitantly initiate at 0.5 mg before each meal and limit total daily dose to 4 mg (7)
- •
- Cyclosporine: Limit daily dose of repaglinide tablets to 6 mg and increase frequency of glucose monitoring when co-administered (7)
- •
- CYP2C8 and CYP3A4 Inhibitors and Drugs That May Increase the Risk of Hypoglycemia: Co-administration may require repaglinide tablet dose reductions and increased frequency of glucose monitoring (7)
- •
- CYP2C8 and CYP3A4 Inducers and Drugs That May Decrease the Blood Glucose Lowering Effect of Repaglinide tablets: Co-administration may require repaglinide tablet dose increases and increased frequency of glucose monitoring (7)
- •
- Drugs That May Blunt Signs and Symptoms of Hypoglycemia: Increased frequency of glucose monitoring may be required when co-administered (7)
USE IN SPECIFIC POPULATIONS
- •
- Lactation: Repaglinide tablets are not recommended when breastfeeding (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage and Administration
2.2 Patients with Severe Renal Impairment
2.3 Dose Modifications for Drug Interactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypoglycemia
5.2 Serious Cardiovascular Adverse Reactions with Concomitant Use with NPH-insulin
5.3 Macrovascular Outcomes
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Monotherapy Trials
14.2 Combination Trials
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage and Administration
The recommended starting dose for patients whose HbA1c is less than 8% is 0.5 mg orally before each meal. For patients whose HbA1c is 8% or greater the starting dose is 1 or 2 mg orally before each meal.
The recommended dose range is 0.5 mg to 4 mg before meals, with a maximum daily dose of 16 mg. The patient’s dose should be doubled up to 4 mg with each meal until satisfactory glycemic control is achieved. At least one week should elapse to assess response after each dose adjustment.
Instruct patients to take repaglinide tablets within 30 minutes before meals. Repaglinide tablets may be dosed 2, 3, or 4 times a day in response to changes in the patient’s meal pattern.
In patients who skip meals, instruct patients to skip the scheduled dose of repaglinide tablets to reduce the risk of hypoglycemia. In patients who experience hypoglycemia, the dose of repaglinide tablets should be reduced [see Warnings and Precautions (5.1)].
2.2 Patients with Severe Renal Impairment
In patients with severe renal impairment (CrCl = 20 – 40 mL/min) initiate repaglinide tablets 0.5 mg orally before each meal. Gradually titrate the dose, if needed to achieve glycemic control.
2.3 Dose Modifications for Drug Interactions
Dosage adjustments are recommended in patients taking concomitant strong CYP3A4 or CYP2C8 inhibitors or strong CYP3A4 or CYP2C8 inducers [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
Concomitant use with gemfibrozil is contraindicated [see Contraindications (4)].
Avoid concomitant use of repaglinide tablets with clopidogrel. If concomitant use cannot be avoided, initiate repaglinide tablets at 0.5 mg before each meal and do not exceed a total daily dose of 4 mg [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
Do not exceed a total daily dose of 6 mg of repaglinide tablets in patients receiving cyclosporine [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
-
3 DOSAGE FORMS AND STRENGTHS
- •
- 0.5 mg tablets (white, round tablets with beveled edges on both sides, debossed with “P240” on one side)
- •
- 1 mg tablets (yellow, round tablets with beveled edges on both sides, debossed with “P241” on one side)
- •
- 2 mg tablets (pink, round tablets with beveled edges on both sides, debossed with “P242” on one side)
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypoglycemia
All glinides, including repaglinide tablets, can cause hypoglycemia [see Adverse Reactions (6.1)]. Severe hypoglycemia can cause seizures, may be life-threatening, or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place an individual and others at risk in situations where these abilities are important (e.g., driving or operating other machinery).
Hypoglycemia can happen suddenly and symptoms may differ in each individual and change over time in the same individual. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes, in patients with diabetic nerve disease, in patients using medications that block the sympathetic nervous system (e.g., beta-blockers) [see Drug Interactions (7)], or in patients who experience recurrent hypoglycemia.
Factors which may increase the risk of hypoglycemia include changes in meal pattern (e.g., macronutrient content), changes in level of physical activity, changes to co-administered medication [see Drug Interactions (7)], and concomitant use with other antidiabetic agents. Patients with renal or hepatic impairment may be at higher risk of hypoglycemia [see Use in Specific Populations (8.6, 8.7)].
Patients should administer repaglinide tablets before meals and be instructed to skip the dose of repaglinide tablets if a meal is skipped. In patients who experience hypoglycemia, the dose of repaglinide tablets should be reduced [see Dosage and Administration (2.1)]. Patients and caregivers must be educated to recognize and manage hypoglycemia. Self-monitoring of blood glucose plays an essential role in the prevention and management of hypoglycemia. In patients at higher risk for hypoglycemia and patients who have reduced symptomatic awareness of hypoglycemia, increased frequency of blood glucose monitoring is recommended.
5.2 Serious Cardiovascular Adverse Reactions with Concomitant Use with NPH-insulin
Across seven controlled trials, there were six serious adverse events of myocardial ischemia in patients treated with repaglinide tablets plus NPH-insulin from two studies, and one event in patients using insulin formulations alone from another study [See Adverse Reactions (6.1)]. Repaglinide tablets are not indicated for use in combination with NPH-insulin.
-
6 ADVERSE REACTIONS
The following serious adverse reaction is also described elsewhere in the labeling:
Hypoglycemia [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying designs, the adverse reaction rates reported in one clinical trial may not be easily compared to those rates reported in another clinical trial, and may not reflect the rates actually observed in clinical practice.
Repaglinide tablets have been administered to 2931 individuals during clinical trials. Approximately 1500 of these individuals with type 2 diabetes have been treated for at least 3 months, 1000 for at least 6 months, and 800 for at least 1 year. The majority of these individuals (1228) received repaglinide tablets in one of five 1-year, active-controlled trials. Over one year, 13% of repaglinide tablets patients were discontinued due to adverse reactions. The most common adverse reactions leading to withdrawal were hyperglycemia, hypoglycemia, and related symptoms.
Table 1 lists the common adverse reactions for repaglinide tablets patients compared to placebo in trials 12 to 24 weeks duration.
Table 1: Adverse Reactions (%) occurring ≥ 2% in Repaglinide Tablets Treated Patients from Pool of 12 to 24 Week Placebo Controlled Trials*
Repaglinide Tablets
N=352Placebo
N=108Upper Respiratory Infection
16
8
Headache
11
10
Sinusitis
6
2
Arthralgia
6
3
Nausea
5
5
Diarrhea
5
2
Back Pain
5
4
Rhinitis
3
3
Constipation
3
2
Vomiting
3
3
Paresthesia
3
3
Chest pain
3
1
Bronchitis
2
1
Dyspepsia
2
2
Urinary tract infection
2
1
Tooth disorder
2
0
Allergy
2
0
*See trial descriptions in Clinical Trials (14)
Hypoglycemia
In clinical trials with repaglinide tablets, hypoglycemia is the most commonly observed adverse reaction. Mild or moderate hypoglycemia occurred in 31% of repaglinide tablet treated patients and 7% of placebo treated patients [see Warnings and Precautions (5.1)].
Hypoglycemia was reported in 16% of 1228 repaglinide tablet patients, 20% of 417 glyburide patients, and 19% of 81 glipizide patients in 1- year controlled trials. Of repaglinide tablet -treated patients with symptomatic hypoglycemia, none developed coma or required hospitalization.
In a 24-week placebo controlled trial, patients who were naïve to oral hypoglycemic agent therapy and patients with a HbA1c below 8% at baseline had a higher frequency of hypoglycemia.
Weight Gain
There was no average gain in body weight when patients previously treated with oral hypoglycemic agents were switched to repaglinide tablets. The average weight gain in patients treated with repaglinide tablets and not previously treated with sulfonylurea drugs was 3.3%.
Cardiovascular Events
The incidence of total serious cardiovascular adverse events, including ischemia, was higher for repaglinide tablets (51/1228 or 4%) than for sulfonylurea drugs (13/498 or 3%) in controlled comparator clinical trials.
Table 2: Summary of Serious Cardiovascular Events in Trials Comparing Repaglinide Tablets to Sulfonylureas (% of total patients with events)
Repaglinide Tablets
SU*
Total Exposed
1228
498
Serious CV Events
4%
3%
Cardiac Ischemic Events
2%
2%
Deaths due to CV Events
0.5%
0.4%
*: glyburide and glipizide
Seven controlled clinical trials included repaglinide tablet combination therapy with NPH-insulin (n=431), insulin formulations alone (n=388) or other combinations (sulfonylurea plus NPH-insulin or repaglinide tablets plus metformin) (n=120). There were six serious adverse events of myocardial ischemia in patients treated with repaglinide tablets plus NPH-insulin from two studies, and one event in patients using insulin formulations alone from another study [see Warnings and Precautions (5.3)].
Combination Therapy with Thiazolidinediones
Hypoglycemia
During 24-week treatment clinical trials of repaglinide tablets -rosiglitazone or repaglinide tablets -pioglitazone combination therapy (a total of 250 patients in combination therapy), hypoglycemia (blood glucose < 50 mg/dL) occurred in 7% of patients in combination therapy compared to 7% for repaglinide tablets monotherapy, and 2% for thiazolidinedione monotherapy.
Peripheral Edema and Heart Failure
Peripheral edema was reported in 12 out of 250 (4.8%) repaglinide tablets -thiazolidinedione combination therapy patients and 3 out of 124 (2.4%) thiazolidinedione monotherapy patients, with no cases reported in these trials for repaglinide tablets monotherapy. There were reports in 2 of 250 patients (0.8%) treated with repaglinide tablets -thiazolidinedione therapy of episodes of edema with congestive heart failure. Both patients had a prior history of coronary artery disease and recovered after treatment with diuretic agents. No comparable cases in the monotherapy treatment groups were reported.
Weight Gain
Mean weight increases associated with combination, repaglinide tablets and pioglitazone therapy were 5.5 kg, 0.3 kg, and 2.0 kg respectively. Mean weight increases associated with combination, repaglinide tablets and rosiglitazone therapy were 4.5 kg, 1.3 kg, and 3.3 kg respectively.
Infrequent Adverse Events (<1% of Patients)
Less common adverse clinical or laboratory events observed in clinical trials included elevated liver enzymes, thrombocytopenia, leukopenia, and anaphylactoid reactions.
6.2 Postmarketing Experience
The following additional adverse reactions have been identified during post approval use of repaglinide tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or a causal relationship to drug exposure.
- •
- Alopecia
- •
- Hemolytic anemia
- •
- Pancreatitis
- •
- Stevens-Johnson Syndrome
- •
- Severe hepatic dysfunction including jaundice and hepatitis
-
7 DRUG INTERACTIONS
Clinically Important Drug Interactions with Repaglinide Tablets
Table 3 includes a list of drugs with clinically important drug interactions when administered concomitantly with repaglinide tablets and instructions for preventing or managing them.
Table 3: Clinically Important Drug Interactions with Repaglinide Tablets
Gemfibrozil
Clinical Impact:
Gemfibrozil significantly increased repaglinide exposures by 8.1 fold [see Clinical Pharmacology (12.3)]
Intervention:
Do not administer repaglinide tablets to patients receiving gemfibrozil [see Contraindications (4)].
Clopidogrel
Clinical Impact:
Clopidogrel increased repaglinide exposures by 3.9-5.1 fold [see Clinical Pharmacology (12.3)]
Intervention:
Avoid concomitant use of repaglinide tablets with clopidogrel. If concomitant use cannot be avoided, initiate repaglinide tablets at 0.5 mg before each meal and do not exceed a total daily dose of 4 mg [see DOSAGE AND ADMINISTRATION (2.3)]. Increased frequency of glucose monitoring may be required during concomitant use.
Cyclosporine
Clinical Impact:
Cyclosporine increased low dose repaglinide exposures by 2.5 fold [see Clinical Pharmacology (12.3)]
Intervention:
Daily maximum repaglinide tablets dose should be limited to 6 mg, and increased frequency of glucose monitoring may be required when repaglinide tablets is co-administered with cyclosporine.
CYP2C8 and CYP3A4 Inhibitors
Intervention:
Repaglinide tablet dose reductions and increased frequency of glucose monitoring may be required when co-administered.
Examples:
Drugs that are known to inhibit CYP3A4 include antifungal agents (ketoconazole, itraconazole) and antibacterial agents (clarithromycin, erythromycin). Drugs that are known to inhibit CYP2C8 include trimethoprim, gemfibrozil, montelukast, deferasirox, and clopidiogrel.
CYP2C8 and CYP3A4 Inducers
Intervention:
Repaglinide tablet dose increases and increased frequency of glucose monitoring may be required when co-administered
Examples:
Drugs that induce the CYP3A4 and/or 2C8 enzyme systems include rifampin, barbiturates, and carbamezapine
Drugs That May Increase the Risk of Hypoglycemia
Intervention:
Repaglinide tablet dose reductions and increased frequency of glucose monitoring may be required when co-administered.
Examples:
Antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, nonsteroidal anti-inflammatory agents (NSAIDs), pentoxifylline, pramlintide, propoxyphene, salicylates, somatostatin analogs (e.g., octreotide), and sulfonamide antibiotics
Drugs That May Decrease the Blood Glucose Lowering Effect of Repaglinide Tablets
Intervention:
Repaglinide tablet dose increases and increased frequency of glucose monitoring may be required when co-administered.
Examples:
Atypical antipsychotics (e.g., olanzapine and clozapine), calcium channel antagonists, corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones.
Drugs That May Blunt Signs and Symptoms of Hypoglycemia
Intervention:
Increased frequency of glucose monitoring may be required when repaglinide tablets are co-administered with these drugs.
Examples:
beta-blockers, clonidine, guanethidine, and reserpine
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited available data from case reports and case series with repaglinide tablets use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy (see Clinical Considerations). Teratogenicity was not observed in rats and rabbits administered repaglinide during organogenesis at approximately 60 and 1 times the maximum daily clinical dose, based on body surface area. No adverse developmental effects were observed in offspring of rats administered repaglinide during late gestation and lactation at approximately 4 times the maximum daily clinical dose (see Data).
The estimated background risk of major birth defects is 6-10% in women with pre-gestational diabetes with a HbA1c>7 and has been reported to be as high as 20-25% in women with a HbA1c>10. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, pre-eclampsia, spontaneous abortions, preterm delivery, and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, stillbirth and macrosomia related morbidity.
Data
Animal Data
Repaglinide was not teratogenic in rats or rabbits at doses 60 times (rats) and approximately 1 times (rabbit) clinical exposure (on a mg/m2 basis) when administered during the period of organogenesis. Offspring of rat dams exposed to repaglinide at ≥22 times clinical exposure on a mg/m2 basis during days 17 to 22 of gestation and during lactation were less viable and developed skeletal deformations consisting of shortening, thickening, and bending of the humerus during the postnatal period. This effect was not seen at doses up to 4 times clinical exposure (on a mg/ m2 basis).
8.2 Lactation
Risk Summary
There are no data on the presence of repaglinide in human milk, the effects on the breastfeeding infant, or the effects on milk production. The drug is present in animal milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk (see Data). Because of the potential for hypoglycemia in breastfed infants, repaglinide tablets are not recommended for use when breastfeeding.
Data
In rat reproduction studies, measurable levels of repaglinide were detected in the breast milk of the dams and lowered blood glucose levels were observed in the pups. Cross fostering studies indicated that skeletal changes [see Use in Specific Populations (8.1)] could be induced in control pups nursed by treated dams, although this occurred to a lesser degree than those pups treated in utero.
8.5 Geriatric Use
In clinical studies of 24 weeks or greater duration, 415 patients were over 65 years of age and no patients were greater than 75 years of age. In one-year, active-controlled trials, no differences were seen in effectiveness or adverse events between these subjects and those less than 65. There was no increase in frequency or severity of hypoglycemia in older subjects, but greater sensitivity of some older individuals to repaglinide tablet therapy cannot be ruled out.
8.6 Renal Impairment
Pharmacokinetic studies of repaglinide were conducted in patients with mild to moderate renal function impairment (CrCl = 40 – 80 mL/min), and severe renal function impairment (CrCl = 20 – 40 mL/min). Initial dose adjustment is not required in patients with mild to moderate renal dysfunction. However, patients with severe renal function impairment should initiate repaglinide tablet therapy with the 0.5 mg dose and be carefully titrated [see Dosage and Administration (2.2)].
Studies were not conducted in patients with creatinine clearances below 20 mL/min or patients with renal failure requiring hemodialysis.
8.7 Hepatic Impairment
A single-dose study was conducted 12 patients with chronic liver disease. Patients with moderate to severe impairment of liver function had higher and more prolonged serum concentrations. Therefore, repaglinide tablets should be used cautiously in patients with impaired liver function. Longer intervals between dose adjustments may be needed to allow full assessment of response.
-
10 OVERDOSAGE
Severe hypoglycemic reactions with coma, seizure, or other neurological impairment may occur and constitute medical emergencies requiring immediate hospitalization. Hypoglycemic symptoms without loss of consciousness or neurologic findings should be treated aggressively with oral glucose and adjustments in drug dosage and/or meal patterns. Close monitoring may continue until the physician is assured that the patient is out of danger. Patients should be closely monitored for a minimum of 24 to 48 hours, since hypoglycemia may recur after apparent clinical recovery. There is no evidence that repaglinide tablets are dialyzable using hemodialysis.
-
11 DESCRIPTION
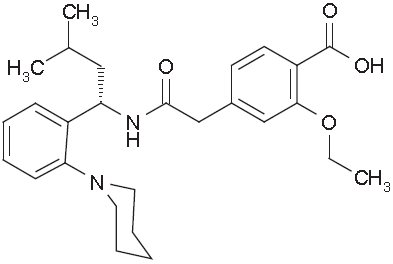
Repaglinide Tablets, USP are an oral blood glucose-lowering drug of the glinide class. Repaglinide, S(+)2-ethoxy-4(2((3-methyl-1-(2-(1-piperidinyl) phenyl)-butyl) amino)-2-oxoethyl) benzoic acid, is chemically unrelated to the oral sulfonylurea insulin secretagogues.
Structural Formula of Repaglinide
Repaglinide is a white to off-white powder with molecular formula C27H36N2O4 and a molecular weight of 452.6. Repaglinide Tablets, USP contain 0.5 mg, 1 mg or 2 mg of repaglinide. In addition, each tablet contains the following inactive ingredients: anhydrous dibasic calcium phosphate, microcrystalline cellulose, corn starch, polacrilin potassium, povidone, glycerin, magnesium stearate, meglumine and poloxamer, and colloidal silicon dioxide. The 1 mg and 2 mg tablets contain ferric oxides (yellow and red, respectively) as coloring agents.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Repaglinide lowers blood glucose levels by stimulating the release of insulin from the pancreas. This action is dependent upon functioning beta (ß) cells in the pancreatic islets. Insulin release is glucose-dependent and diminishes at low glucose concentrations.
Repaglinide closes ATP-dependent potassium channels in the ß-cell membrane by binding at characterizable sites. This potassium channel blockade depolarizes the ß-cell, which leads to an opening of calcium channels. The resulting increased calcium influx induces insulin secretion. The ion channel mechanism is highly tissue selective with low affinity for heart and skeletal muscle.
12.2 Pharmacodynamics
A four-week, double-blind, placebo-controlled dose-response trial was conducted in 138 patients with type 2 diabetes using doses ranging from 0.25 (not an approved dose) to 4 mg taken with each of three meals. Repaglinide tablet therapy resulted in dose-proportional glucose lowering over the full dose range. Plasma insulin levels increased after meals and reverted toward baseline before the next meal. Most of the fasting blood glucose-lowering effect was demonstrated within 1-2 weeks.
In a double-blind, placebo-controlled, 3-month dose titration study, repaglinide tablets or placebo doses for each patient were increased weekly from 0.25 mg (not an approved dose) through 0.5, 1, and 2 mg, to a maximum of 4 mg, until a fasting plasma glucose (FPG) level <160 mg/dL was achieved or the maximum dose reached. The dose that achieved the targeted control or the maximum dose was continued to end of study. FPG and 2-hour post-prandial glucose (PPG) increased in patients receiving placebo and decreased in patients treated with repaglinide. Differences between the repaglinide- and placebo-treated groups were -61 mg/dL (FPG) and -104 mg/dL (PPG) (Table 4).
Table 4: Repaglinide Tablets vs Placebo
Mean Change from Baseline after 3 Months of Treatment
Repaglinide
Placebo
N
66
33
Fasting Plasma Glucose (mg/dL)
- Baseline
220.2
215.3
- Change from baseline
(at last visit)
-31.0*
30.3Post Prandial Glucose (mg/dL)
- Baseline
261.7
245.2
- Change from baseline
(at last visit)
-47.6*
56.5*: p< 0.05 for between group difference
The dosing of repaglinide tablets relative to meal-related insulin release was studied in three trials including 58 patients. Glycemic control was maintained during a period in which the meal and dosing pattern was varied (2, 3 or 4 meals per day; before meals x 2, 3, or 4) compared with a period of 3 regular meals and 3 doses per day (before meals x 3). Blood glucose-lowering effect did not differ when repaglinide tablets were administered at the start of a meal, 15 minutes before, or 30 minutes before the meal.
12.3 Pharmacokinetics
The pharmacokinetic parameters of repaglinide obtained from a single-dose, crossover study in healthy subjects and from a multiple-dose, parallel, dose-proportionality (0.5, 1, 2 and 4 mg) study in patients with type 2 diabetes are summarized in Tables 5 and 6. These data indicate that repaglinide did not accumulate in serum. Clearance of oral repaglinide did not change over the 0.5 - 4 mg dose range, indicating a linear relationship between dose and plasma drug levels.
Table 5: Pharmacokinetic Parameters for Repaglinide in Healthy Subjects
Parameter
CL (based on i.v.)
38 ± 16 L/hr
Vss (based on i.v.)
31 ± 12 L
AbsBio
56 ± 9%
CL = total body clearance
Vss = volume of distribution at steady state
AbsBio = absolute bioavailability
Table 6: Pharmacokinetic Parameters for Repaglinide in Patients with Type 2 Diabetes*
Pharmacokinetic Parameter
Dose
(mg)AUC0-24 hr
(ng/mL*hr)
Mean (SD)Cmax0-5 hr
(ng/mL)
Mean (SD)0.5
68.9 (154.4)
9.8 (10.2)
1
125.8 (129.8)
18.3 (9.1)
2
152.4 (89.60)
26.0 (13.0)
4
447.4 (211.3)
65.8 (30.1)
Tmax0-5 hr
Means (SD)
T½
Means (Ind Range)
0.5 - 4
1.0 – 1.4 (0.3 – 0.5) hr
1.0 – 1.4 (0.4 – 8.0) hr
*dosed preprandially with three meals
Absorption
After oral administration, repaglinide is completely absorbed from the gastrointestinal tract. After single and multiple oral doses in healthy subjects or in patients, peak plasma drug levels (Cmax ) occur within 1 hour (Tmax ). Repaglinide is eliminated from the blood stream with a half-life of approximately 1 hour. The mean absolute bioavailability is 56%. When repaglinide was given with food, the mean Tmax was not changed, but the mean Cmax and AUC (area under the time/plasma concentration curve) were decreased 20% and 12.4%, respectively.
Distribution
After intravenous (IV) dosing in healthy subjects, the volume of distribution at steady state (Vss) was 31 L, and the total body clearance (CL) was 38 L/h. Protein binding and binding to human serum albumin was greater than 98%.
Metabolism and Elimination
Repaglinide is completely metabolized by oxidative biotransformation and direct conjugation with glucuronic acid after either an IV or oral dose. The major metabolites are an oxidized dicarboxylic acid (M2), the aromatic amine (M1), and the acyl glucuronide (M7). The cytochrome P-450 enzyme system, specifically 2C8 and 3A4, have been shown to be involved in the N-dealkylation of repaglinide to M2 and the further oxidation to M1. Metabolites do not contribute to the glucose-lowering effect of repaglinide. Within 96 hours after dosing with 14C-repaglinide as a single, oral dose, approximately 90% of the radiolabel was recovered in the feces and approximately 8% in the urine. Only 0.1% of the dose is cleared in the urine as parent compound. The major metabolite (M2) accounted for 60% of the administered dose. Less than 2% of parent drug was recovered in feces. Repaglinide appears to be a substrate for active hepatic uptake transporter (organic anion transporting protein OATP1B1).
Variability of Exposure
Repaglinide AUC after multiple doses of 0.25 to 4 mg with each meal varies over a wide range. The intra-individual and inter-individual coefficients of variation were 36% and 69%, respectively. AUC over the therapeutic dose range included 69 to 1005 ng/mL*hr, but AUC exposure up to 5417 ng/mL*hr was reached in dose escalation studies without apparent adverse consequences.
Specific Populations
Geriatric
Healthy volunteers were treated with a regimen of 2 mg repaglinide tablets taken before each of 3 meals. There were no significant differences in repaglinide pharmacokinetics between the group of patients <65 years of age and comparably sized group of patients ≥65 years of age [see Use in Specific Populations (8.5)].
Gender
A comparison of pharmacokinetics in males and females showed the AUC over the 0.5 mg to 4 mg dose range to be 15% to 70% higher in females with type 2 diabetes. This difference was not reflected in the frequency of hypoglycemic episodes (male: 16%; female: 17%) or other adverse events.
Race
No pharmacokinetic studies to assess the effects of race have been performed, but in a U.S. 1-year study in patients with type 2 diabetes, the blood glucose-lowering effect was comparable between Caucasians (n=297) and African-Americans (n=33). In a U.S. dose-response study, there was no apparent difference in exposure (AUC) between Caucasians (n=74) and Hispanics (n=33).
Renal Impairment
Single-dose and steady-state pharmacokinetics of repaglinide were compared between patients with type 2 diabetes and normal renal function (CrCl > 80 mL/min), mild to moderate renal function impairment (CrCl = 40 – 80 mL/min), and severe renal function impairment (CrCl = 20 – 40 mL/min). Both AUC and Cmax of repaglinide were similar in patients with normal and mild to moderately impaired renal function (mean values 56.7 ng/mL*hr vs 57.2 ng/mL*hr and 37.5 ng/mL vs 37.7 ng/mL, respectively.) Patients with severely reduced renal function had elevated mean AUC and Cmax values (98.0 ng/mL*hr and 50.7 ng/mL, respectively), but this study showed only a weak correlation between repaglinide levels and creatinine clearance.
Hepatic Impairment
A single-dose, open-label study was conducted in 12 healthy subjects and 12 patients with chronic liver disease (CLD) classified by Child-Pugh scale and caffeine clearance. Patients with moderate to severe impairment of liver function had higher and more prolonged serum concentrations of both total and unbound repaglinide than healthy subjects (AUChealthy : 91.6 ng/mL*hr; AUCCLD patients : 368.9 ng/mL*hr; Cmax, healthy : 46.7 ng/mL; Cmax, CLD patients : 105.4 ng/mL). AUC was statistically correlated with caffeine clearance. No difference in glucose profiles was observed across patient groups.
Drug-Drug Interactions
Drug interaction studies performed in healthy volunteers show that repaglinide tablets had no clinically relevant effect on the pharmacokinetic properties of digoxin, theophylline, or warfarin. Co-administration of cimetidine with repaglinide tablets did not significantly alter the absorption and disposition of repaglinide.
Additionally, the following drugs were studied in healthy volunteers with co-administration of repaglinide tablets.
Table 7: Effect of Other Drugs on AUC and Cmax of Repaglinide
Study Drug
Dosing
- Repaglinide
- Dosing1
Dosing1
Repaglinide
AUC
Cmax
Clarithromycin*
- 250 mg BID
for 4 days
- 40% ↑
- 67% ↑
Clopidogrel*
- 300 mg
- (Day 1)
75 mg QD (Day 2-3)
(Day 1)
75 mg QD (Day 2-3)0.25 mg
(Day 1and 3)
- (day 1) 5.1
fold ↑
(3.9-6.6)
(day 3) 3.9
fold ↑
(2.9-5.3)
- 2.5 fold ↑
(1.8-3.5)
2.0 fold ↑
(1.3-3.1)
Cyclosporine
- 100 mg
(2 doses 12 hours apart)
- 2.5 fold ↑
- 1.8 fold ↑
Deferasirox*
- 30 mg/kg QD
for 4 days
0.5 mg
- 2.3 fold ↑
- 62% ↑
Fenofibrate
- 200 mg QD
for 5 days
- 0%
- 0%
Gemfibrozil*
- 600 mg BID
for 3 days
- 8.1 fold ↑
- 2.4 fold ↑
Itraconazole*
- 100 mg BID
for 3 days
- 1.4 fold ↑
- 1.5 fold ↑
Gemfibrozil + Itraconazole*
Co-administration- Gem: 600 mg BID
for 3 days
Itra: 100 mg BID
for 3 days
- 19 fold ↑
- 2.8 fold ↑
Ketoconazole
- 200 mg QD
for 4 days
2 mg
- 15% ↑
- 16% ↑
Levonorgestrel/
ethinyl Estradiol- (0.15 mg/0.03 mg)
Combination tablet QD
for 21 days
2 mg
- 0%
- 20% ↑
Nifedipine*
- 10 mg TID
for 4 days
2 mg
- 0%
- 0%
Rifampin*
- 600 mg QD
for 6-7 days
4 mg
- 32 - 80% ↓
- 17 - 79% ↓
Simvastatin
- 20 mg QD
for 4 days
2 mg
- 0%
- 26% ↑
Trimethoprim*
- 160 mg BID
for 2 days
160 mg QD
for 1 day
- 61% ↑
- 41% ↑
1 Unless indicated all drug interactions were observed with single dose of 0.25 mg repaglinide
↑ indicates increase
↓ indicates decrease
* Indicates data are from published literature
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 104-week carcinogenicity study in rats at doses up to 120 mg/kg/day, which is approximately 90 times clinical exposure on a mg/m2 basis, the incidences of benign adenomas of the thyroid and liver were increased in male rats. No evidence of carcinogenicity was found in female rats. The higher incidences of thyroid and liver tumors in male rats were not seen at lower dose of 30 mg/kg/day and 60 mg/kg/day respectively (which are over 20 and 45 times, respectively, clinical exposures on a mg/m2 basis). In a 104-week carcinogenicity study in mice at doses up to 500 mg/kg/day, no evidence of carcinogenicity was found in mice (which is approximately 187 times clinical exposure on a mg/m2 basis).
Repaglinide was non-genotoxic in a battery of in vivo and in vitro studies: Bacterial mutagenesis (Ames test), in vitro forward cell mutation assay in V79 cells (HGPRT), in vitro chromosomal aberration assay in human lymphocytes, unscheduled and replicating DNA synthesis in rat liver, and in vivo mouse and rat micronucleus tests.
In a rat fertility study, repaglinide was administered to male and female rats at doses up to 300 and 80 mg/kg/day, respectively. No adverse effects on fertility were observed (which are over 60 times clinical exposure on a mg/m2 basis).
-
14 CLINICAL STUDIES
14.1 Monotherapy Trials
A double-blind, placebo-controlled trial was carried out in 362 patients treated for 24 weeks. HbA1c for the repaglinide- treated groups (1 and 4 mg groups combined) at the end of the study was decreased compared to the placebo-treated group in treatment naïve patients and in patients previously treated with oral hypoglycemic agents by 2.1% and 1.7%, respectively. In this fixed-dose trial, patients who were treatment naïve to oral hypoglycemic agent therapy and patients with a HbA1c below 8% at baseline showed greater blood glucose-lowering.
14.2 Combination Trials
Repaglinide Tablets in Combination With Metformin
Repaglinide tablets were studied in combination with metformin in 83 patients not satisfactorily controlled on exercise, diet, and metformin alone. Repaglinide tablet dosage was titrated for 4 to 8 weeks, followed by a 3-month maintenance period. Combination therapy with repaglinide tablets and metformin resulted in statistically significant improvement in HbA1c and fasting plasma glucose (FPG) compared to repaglinide tablets or metformin monotherapy (Table 8). In this study where metformin dosage was kept constant, the combination therapy of repaglinide tablets and metformin showed dose-sparing effects with respect to repaglinide tablets. The improvement in HbA1c and FPG of the combination group was achieved at a lower daily repaglinide tablet dosage than in the repaglinide tablets monotherapy group (Table 8).
Table 8: Repaglinide Tablets in Combination with Metformin:
Mean Change from Baseline after 4 to 5 Months of Treatment1- Repaglinide Tablet
Monotherapy
- Repaglinide Tablet
Combination Therapy
with Metformin
- Metformin
Monotherapy
N
- 28
- 27
- 27
Median Final Dose (mg/day)
- 12
- 6 (Repaglinide Tablets)
1500 (metformin)
- 1500
HbA1c (%)
- Baseline
- 8.6
- 8.3
- 8.6
- Change from baseline
- -0.38
- -1.41*
- -0.33
Fasting Plasma Glucose (mg/dL)
- Baseline
- 174
- 184
- 194
- Change from baseline
- 8.8
- -39.2*
- -4.5
Weight (kg)
- Baseline
- 87
- 93
- 91
- Change from baseline
- 3.0
- 2.4#
- -0.90
1: based on intent-to-treat analysis
*: p< 0.05, for pairwise comparisons with repaglinide tablets and metformin monotherapy.
#: p< 0.05, for pairwise comparison with metformin.
Repaglinide Tablets in Combination With Pioglitazone
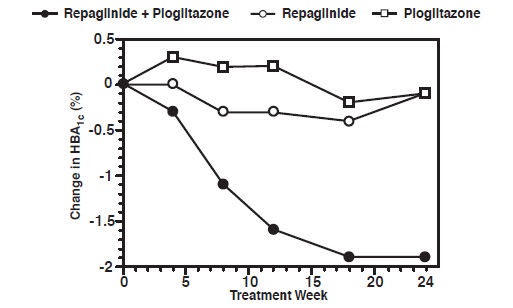
A combination therapy regimen of repaglinide tablets and pioglitazone (N=123) was compared to repaglinide tablets alone (N=61) and pioglitazone alone (N=62) in a 24-week trial that enrolled 246 patients previously treated with sulfonylurea or metformin monotherapy (HbA1c > 7.0%). Repaglinide tablets dosage was titrated during the first 12 weeks, followed by a 12-week maintenance period. Combination therapy resulted in statistically significant improvement in HbA1c and FPG compared to monotherapy (Figure 1). The changes from baseline for completers in FPG (mg/dL) and HbA1c (%), respectively were: -39.8 mg/dL and -0.1% for repaglinide tablets, -35.3 mg/dL and -0.1% for pioglitazone and -92.4 mg/dL and -1.9% for the combination. In this study where pioglitazone dosage was kept constant, the combination therapy group showed dose-sparing effects with respect to repaglinide tablets (see Figure 1 Legend). The improvement in HbA1c and FPG of the combination group was achieved at a lower daily repaglinide tablet dosage than in the repaglinide tablet monotherapy group.
Figure 1: Repaglinide Tablets in Combination with Pioglitazone: HbA1c Values
LEGEND: HbA1c values by study week for patients who completed study (combination, N = 101; Repaglinide, N = 35, pioglitazone, N = 26).
Subjects with FPG above 270 mg/dL were withdrawn from the study.
Pioglitazone dose: fixed at 30 mg/day; repaglinide median final dose: 6 mg/day for combination and 10 mg/day for monotherapy.
Repaglinide Tablets in Combination With Rosiglitazone
A combination therapy regimen of repaglinide tablets and rosiglitazone was compared to monotherapy with either agent alone in a 24-week trial that enrolled 252 patients previously treated with sulfonylurea or metformin (HbA1c > 7.0%). Combination therapy resulted in statistically significant improvement in HbA1c and FPG compared to monotherapy (Table 9 below). The glycemic effects of the combination therapy were dose-sparing with respect to both total daily repaglinide tablet dosage and total daily rosiglitazone dosage (see Table 9 Legend). The improvement in HbA1c and FPG of the combination therapy group was achieved with lower daily dose of repaglinide tablets and rosiglitazone, as compared to the respective monotherapy groups.
Table 9: Repaglinide Tablets in Combination with Rosiglitazone:
Mean Change from Baseline in a 24-Week Study1-
Repaglinide Tablet
Monotherapy
-
Repaglinide Tablet
Combination Therapy
with Rosiglitazone
-
Rosiglitazone
Monotherapy
N
- 63
- 127
- 62
Median Final Dose (mg/day)
- 12
- 6 (Repaglinide Tablets)
4 (Rosiglitazone)
- 8
HbA1c (%)
- Baseline
- 9.3
- 9.1
- 9.0
- Change from baseline
- -0.17
- -1.43*
- -0.56
Fasting Plasma Glucose (mg/dL)
- Baseline
- 269
- 257
- 252
- Change from baseline
- -54
- -94*
- -67
Change in Weight (kg)
- +1.3
- +4.5#
- +3.3
1: based on intent-to-treat analysis
*: p ≤ 0.001 for comparison to either monotherapy
#: p < 0.05 for comparison to repaglinide tablets
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Repaglinide Tablets, USP are supplied as round tablets with beveled edges on both sides available in 0.5 mg (white, debossed with “P240” on one side), 1 mg (yellow, debossed with “P241” on one side) and 2 mg (pink, debossed with “P242” on one side) strengths.
- 0.5 mg tablets (white)
- Bottles of 100
- NDC 0574-0240-01
Bottles of 500
- NDC 0574-0240-05
Bottles of 1000
- NDC 0574-0240-10
- 1 mg tablets (yellow)
- Bottles of 100
- NDC 0574-0241-01
Bottles of 500
- NDC 0574-0241-05
Bottles of 1000
- NDC 0574-0241-10
- 2 mg tablets (pink)
- Bottles of 100
- NDC 0574-0242-01
Bottles of 500
- NDC 0574-0242-05
Bottles of 1000
- NDC 0574-0242-10
Store at 20° to 25° C (68° to 77° F) [see USP Controlled Room Temperature].
Protect from moisture. Keep bottles tightly close.
Dispense in tight containers with safety closures.
-
17 PATIENT COUNSELING INFORMATION
Hypoglycemia
Inform patients that repaglinide tablets can cause hypoglycemia and instruct patients and their caregivers on self-management procedures including glucose monitoring and management of hypoglycemia. Inform patients that their ability to concentrate and react may be impaired as a result of hypoglycemia. In patients at higher risk for hypoglycemia and patients who have reduced symptomatic awareness of hypoglycemia, increased frequency of blood glucose monitoring is recommended [see Warnings and Precautions (5.1)].
Administration
Instruct patients to take repaglinide tablets within 30 minutes before meals. Instruct patients to skip their dose of repaglinide tablets when a meal is skipped. [see Dosage and Administration (2)].
Drug Interactions
Discuss potential drug interactions with patients and inform them of potential drug-drug interactions with repaglinide tablets. [see Drug Interactions (7)].
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL – 0.5 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 1 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 2 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
REPAGLINIDE
repaglinide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0574-0240 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength REPAGLINIDE (UNII: 668Z8C33LU) (REPAGLINIDE - UNII:668Z8C33LU) REPAGLINIDE 0.5 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) POLACRILIN POTASSIUM (UNII: 0BZ5A00FQU) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) GLYCERIN (UNII: PDC6A3C0OX) MAGNESIUM STEARATE (UNII: 70097M6I30) MEGLUMINE (UNII: 6HG8UB2MUY) POLOXAMER 407 (UNII: TUF2IVW3M2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color WHITE Score no score Shape ROUND Size 6mm Flavor Imprint Code P240 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0574-0240-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 08/09/2013 06/30/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA201189 08/09/2013 06/30/2024 REPAGLINIDE
repaglinide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0574-0241 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength REPAGLINIDE (UNII: 668Z8C33LU) (REPAGLINIDE - UNII:668Z8C33LU) REPAGLINIDE 1 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) POLACRILIN POTASSIUM (UNII: 0BZ5A00FQU) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) GLYCERIN (UNII: PDC6A3C0OX) MAGNESIUM STEARATE (UNII: 70097M6I30) MEGLUMINE (UNII: 6HG8UB2MUY) POLOXAMER 407 (UNII: TUF2IVW3M2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color YELLOW Score no score Shape ROUND Size 6mm Flavor Imprint Code P241 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0574-0241-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/22/2014 01/31/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA201189 01/22/2014 01/31/2025 REPAGLINIDE
repaglinide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0574-0242 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength REPAGLINIDE (UNII: 668Z8C33LU) (REPAGLINIDE - UNII:668Z8C33LU) REPAGLINIDE 2 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) POLACRILIN POTASSIUM (UNII: 0BZ5A00FQU) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) GLYCERIN (UNII: PDC6A3C0OX) MAGNESIUM STEARATE (UNII: 70097M6I30) MEGLUMINE (UNII: 6HG8UB2MUY) POLOXAMER 407 (UNII: TUF2IVW3M2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color PINK Score no score Shape ROUND Size 6mm Flavor Imprint Code P242 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0574-0242-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/22/2014 05/31/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA201189 01/22/2014 05/31/2024 Labeler - Padagis US LLC (967694121)