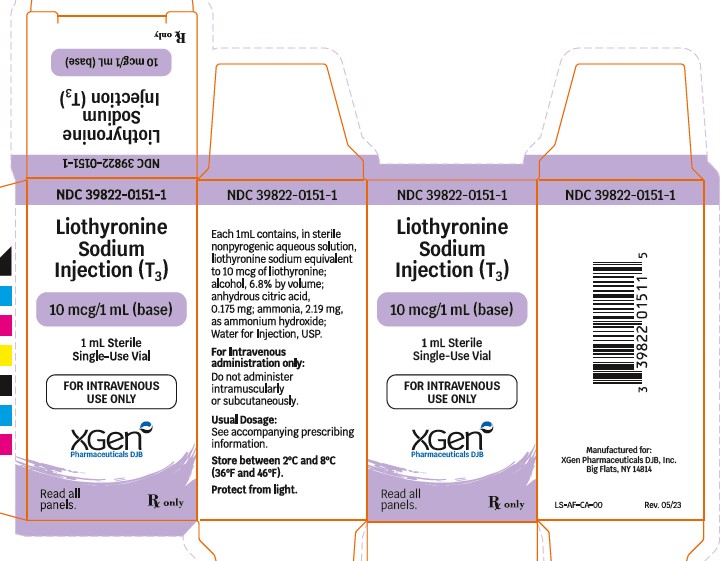
Label: LIOTHYRONINE SODIUM injection, solution
- NDC Code(s): 39822-0151-1
- Packager: XGen Pharmaceuticals DJB, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Thyroid hormone drugs are natural or synthetic preparations containing tetraiodothyronine (T 4, levothyroxine) sodium or triiodothyronine (T 3, liothyronine) sodium or both. T 4 and T 3 are produced in the human thyroid gland by the iodination and coupling of the amino acid tyrosine. T 4 contains four iodine atoms and is formed by the coupling of two molecules of diiodotyrosine (DIT). T 3 contains three atoms of iodine and is formed by the coupling of one molecule of DIT with one molecule of monoiodotyrosine (MIT). Both hormones are stored in the thyroid colloid as thyroglobulin and released into the circulation. The major source of T 3 has been shown to be peripheral deiodination of T 4. T 3 is bound less firmly than T 4 in the serum, enters peripheral tissues more readily, and binds to specific nuclear receptor(s) to initiate hormonal, metabolic effects. T 4 is the prohormone which is deiodinated to T 3 for hormone activity.
Thyroid hormone preparations belong to two categories: (1) natural hormonal preparations derived from animal thyroid, and (2) synthetic preparations. Natural preparations include desiccated thyroid and thyroglobulin. Desiccated thyroid is derived from domesticated animals that are used for food by man (either beef or hog thyroid), and thyroglobulin is derived from thyroid glands of the hog.
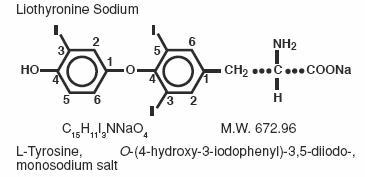
Liothyronine sodium injection (T 3) contains liothyronine (L-triiodothyronine or L-T 3), a synthetic form of a natural thyroid hormone, as the sodium salt.
The structural and empirical formulas and molecular weight of liothyronine sodium are given below.
In euthyroid patients, 25 mcg of liothyronine is equivalent to approximately 1 grain of desiccated thyroid or thyroglobulin and 0.1 mg of L-thyroxine.
Each mL of liothyronine sodium injection (T 3) in amber glass vials contains, in sterile non-pyrogenic aqueous solution, liothyronine sodium equivalent to 10 mcg of liothyronine; alcohol, 6.8% by volume; anhydrous citric acid, 0.175 mg; ammonia, 2.19 mg, as ammonium hydroxide; Water for Injection, USP.
-
CLINICAL PHARMACOLOGY
Thyroid hormones enhance oxygen consumption by most tissues of the body and increase the basal metabolic rate and the metabolism of carbohydrates, lipids and proteins. In vitro studies indicate that T 3 increases aerobic mitochondrial function, thereby increasing the rates of synthesis and utilization of myocardial high-energy phosphates. This, in turn, stimulates myosin ATPase and reduces tissue lactic acidosis. Thus, thyroid hormones exert a profound influence on virtually every organ system in the body and are of particular importance in the development of the central nervous system.
While the source of levothyroxine (T 4) and some triiodothyronine (T 3) is via secretion from the thyroid gland, it is now well-established that approximately 80% of circulating T 3 arises predominantly by way of the extrathyroidal conversion of T 4. The membrane-bound enzyme responsible for this reaction is iodothyronine 5’-deiodinase. Activity of the enzyme is greatest in the liver and kidney. A second pathway of T 4 to T 3 conversion occurs via a PTU-insensitive 5’-deiodinase located primarily in the pituitary and central nervous system.
The prohormone T 4 must be converted to T 3 in the body before it can exert biological effects. During periods of illness or stress, this conversion is often inhibited and can be diverted to the inactive reverse T 3 (rT 3) moiety. Therefore, correction of the hypothyroid condition in patients with myxedema coma is facilitated by the parenteral administration of triiodothyronine (T 3). T 3 is bound much less firmly to serum binding proteins and therefore penetrates into the cells much more rapidly than T 4. Also, the binding of T 3 to a nuclear thyroid hormone receptor seems to initiate most effects of thyroid hormone in tissues. Although most thyroid hormone analogs, both natural and synthetic, will bind to this protein, the affinity of T 3 for this receptor is roughly 10-fold higher than that of T 4. Thus, T 3 is the biologically active thyroid hormone.
Pharmacodynamics
The clinical features of myxedema coma include depression of the cardiovascular, respiratory, gastrointestinal and central nervous systems, impaired diuresis, and hypothermia. Administration of thyroid hormones reverses or attenuates these conditions. Thyroid hormones increase heart rate, ventricular contractility and cardiac output, as well as decrease total systemic vascular resistance. They also increase the rate and depth of respiration, motility of the gastrointestinal tract, rapidity of cerebration, and vasodilatation. Thyroid hormones correct hypothermia by markedly increasing the basal metabolic rate, as well as the number and activity of mitochondria in almost all cells of the body.
Pharmacokinetics
Since liothyronine sodium (T 3) is not firmly bound to serum protein, it is readily available to body tissues.
Liothyronine sodium has a rapid cutoff of activity which permits quick dosage adjustment and facilitates control of the effects of overdosage, should they occur.
The higher affinity of levothyroxine (T 4) as compared to triiodothyronine (T 3) for both thyroid-binding globulin and thyroid-binding prealbumin partially explains the higher serum levels and longer half-life of the former hormone. Both protein-bound hormones exist in reverse equilibrium with minute amounts of free hormone, the latter accounting for the metabolic activity. T 4 is deiodinated to T 3.
A single dose of liothyronine sodium administered intravenously produces a detectable metabolic response in as little as two to four hours and a maximum therapeutic response within two days. However, no pharmacokinetic studies have been performed with intravenous liothyronine (T 3) in myxedema coma or precoma patients.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Thyroid hormone preparations are generally contraindicated in patients with diagnosed but as yet uncorrected adrenal cortical insufficiency or untreated thyrotoxicosis. Thyroid hormone preparations are also generally contraindicated in patients with hypersensitivity to any of the active or extraneous constituents of these preparations; however, there is no well-documented evidence in the literature of true allergic or idiosyncratic reactions to thyroid hormone.
Concomitant use of liothyronine sodium injection (T 3) and artificial rewarming of patients is contraindicated. (See PRECAUTIONS.)
-
BOXED WARNING
(What is this?)
BOXED WARNING
Drugs with thyroid hormone activity, alone or together with other therapeutic agents, have been used for the treatment of obesity. In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction. Larger doses may produce serious or even life-threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects.
-
WARNINGS
The use of thyroid hormones in the therapy of obesity, alone or combined with other drugs, is unjustified and has been shown to be ineffective. Neither is their use justified for the treatment of male or female infertility unless this condition is accompanied by hypothyroidism.
Thyroid hormones should be used with great caution in a number of circumstances where the integrity of the cardiovascular system, particularly the coronary arteries, is suspect. These include patients with angina pectoris or the elderly, in whom there is a greater likelihood of occult cardiac disease. Therefore, in patients with compromised cardiac function, use thyroid hormones in conjunction with careful cardiac monitoring. Although the specific dosage of liothyronine sodium injection (T 3) depends upon individual circumstances, in patients with known or suspected cardiovascular disease the extremely rapid onset of action of liothyronine sodium injection (T 3) may warrant initiating therapy at a dose of 10 mcg to 20 mcg. (See DOSAGE AND ADMINISTRATION.)
Myxedematous patients are very sensitive to thyroid hormones; dosage should be started at a low level and increased gradually as acute changes may precipitate adverse cardiovascular events.
Severe and prolonged hypothyroidism can lead to a decreased level of adrenocortical activity commensurate with the lowered metabolic state. When thyroid replacement therapy is administered, the metabolism increases at a greater rate than adrenocortical activity. This can precipitate adrenocortical insufficiency. Therefore, in severe and prolonged hypothyroidism, supplemental adrenocortical steroids may be necessary.
In rare instances, the administration of thyroid hormone may precipitate a hyperthyroid state or may aggravate existing hyperthyroidism.
Extreme caution is advised when administering thyroid hormones with digitalis or vasopressors. (See PRECAUTIONS-Drug Interactions.)
Fluid therapy should be administered with great care to prevent cardiac decompensation. (See PRECAUTIONS-Adjunctive Therapy.)
-
PRECAUTIONS
General
Thyroid hormone therapy in patients with concomitant diabetes mellitus (see PRECAUTIONS-Drug Interactions, Insulin or Oral Hypoglycemics regarding interaction and dose adjustment with insulin) or insipidus or adrenal cortical insufficiency may aggravate the intensity of their symptoms. Appropriate adjustments of the various therapeutic measures directed at these concomitant endocrine diseases are required.
The therapy of myxedema coma requires simultaneous administration of glucocorticoids. (See PRECAUTIONS-Adjunctive Therapy).
Hypothyroidism decreases and hyperthyroidism increases the sensitivity to anticoagulants. Prothrombin time should be closely monitored in thyroid-treated patients on anticoagulants and dosage of the latter agents adjusted on the basis of frequent prothrombin time determinations.
Oral therapy should be resumed as soon as the clinical situation has been stabilized and the patient is able to take oral medication. If L-thyroxine rather than liothyronine sodium is used in initiating oral therapy, the physician should bear in mind that there is a delay of several days in the onset of L-thyroxine activity and that intravenous therapy should be discontinued gradually.
Adjunctive Therapy
Many investigators recommend that corticosteroids be administered routinely in the initial emergency treatment of all patients with myxedema coma. Patients with pituitary myxedema should receive adrenocortical hormone replacement therapy at or before the start of liothyronine sodium injection (T 3) therapy. Similarly, patients with primary myxedema may also require adrenocortical hormone replacement therapy since a rapid return to normal body metabolism from a severely hypothyroid state may result in acute adrenocortical insufficiency and shock.
In considering the need to elevate blood pressure, it should be kept in mind that tissue metabolic requirements are markedly reduced in the hypothyroid patient. Because arrhythmias and circulatory collapse have infrequently occurred following the concomitant administration of thyroid hormones and vasopressor therapies, use caution when administering these therapies concomitantly. (See PRECAUTIONS-Drug Interactions, Vasopressors.)
Hyponatremia is frequently present in myxedema coma, but usually resolves without specific therapy as the metabolic status of the patient is improved with thyroid hormone treatment. Fluid therapy should be administered with great care to prevent cardiac decompensation. In addition, some patients with myxedema have inappropriate secretion of ADH and are susceptible to water intoxication.
In some patients, respiratory depression has been a significant factor in the development or persistence of the comatose state. Decreased oxygen saturation and elevated CO 2 levels respond quickly to artificial respiration.
Infection is often present in myxedema coma and should be looked for and treated appropriately.
Concomitant use of liothyronine sodium injection (T 3) and artificial rewarming of patients is contraindicated. Although patients in myxedema coma are often hypothermic, most investigators believe that artificial rewarming is of little value or may be harmful. The peripheral vasodilation produced by external heat serves to further decrease circulation to vital internal organs and to increase shock if present. It has been reported that the administration of liothyronine sodium will restore a normal body temperature in 24 to 48 hours if heat loss is prevented by keeping the patient covered with blankets in a warm room.
Laboratory Tests
Treatment of patients with thyroid hormones requires the periodic assessment of thyroid status by means of appropriate laboratory tests besides the full clinical evaluation. Serum T 3 and TSH levels should be monitored to assess dosage adequacy and biologic effectiveness.
Drug Interactions
Oral Anticoagulants: Thyroid hormones appear to increase catabolism of vitamin K-dependent clotting factors. If oral anticoagulants are also being given, compensatory increases in clotting factor synthesis are impaired. Patients stabilized on oral anticoagulants who are found to require thyroid replacement therapy should be watched very closely when thyroid is started. If a patient is truly hypothyroid, it is likely that a reduction in anticoagulant dosage will be required. No special precautions appear to be necessary when oral anticoagulant therapy is begun in a patient already stabilized on maintenance thyroid replacement therapy.
Insulin or Oral Hypoglycemics:Initiating thyroid replacement therapy may cause increases in insulin or oral hypoglycemic requirements. The effects seen are poorly understood and depend upon a variety of factors such as dose and type of thyroid preparations and endocrine status of the patient. Patients receiving insulin or oral hypoglycemics should be closely watched during initiation of thyroid replacement therapy.
Estrogen, Oral Contraceptives:Estrogens tend to increase serum thyroxine-binding globulin (TBG). In a patient with a nonfunctioning thyroid gland who is receiving thyroid replacement therapy, free levothyroxine may be decreased when estrogens are started thus increasing thyroid requirements. However, if the patient’s thyroid gland has sufficient function, the decreased free thyroxine will result in a compensatory increase in thyroxine output by the thyroid. Therefore, patients without a functioning thyroid gland who are on thyroid replacement therapy may need to increase their thyroid dose if estrogens or estrogen-containing oral contraceptives are given.
Tricyclic Antidepressants:Use of thyroid products with imipramine and other tricyclic antidepressants may increase receptor sensitivity and enhance antidepressant activity; transient cardiac arrhythmias have been observed. Thyroid hormone activity may also be enhanced.
Digitalis:Thyroid preparations may potentiate the toxic effects of digitalis. Thyroid hormonal replacement increases metabolic rate, which requires an increase in digitalis dosage.
Ketamine:When administered to patients on a thyroid preparation, this parenteral anesthetic may cause hypertension and tachycardia. Use with caution and be prepared to treat hypertension, if necessary.
Vasopressors:Thyroid hormones increase the adrenergic effect of catecholamines such as epinephrine and norepinephrine. Therefore, use of vasopressors in patients receiving thyroid hormone preparations may increase the risk of precipitating coronary insufficiency, especially in patients with coronary artery disease. Therefore, use caution when administering vasopressors with liothyronine (T 3).
DRUG/LABORATORY TEST INTERACTIONS
The following drugs or moieties are known to interfere with laboratory tests performed in patients on thyroid hormone therapy: androgens, corticosteroids, estrogens, oral contraceptives containing estrogens, iodine-containing preparations and the numerous preparations containing salicylates.
- Changes in TBG concentration should be taken into consideration in the interpretation of T 4 and T 3 values. In such cases, the unbound (free) hormone should be measured. Pregnancy, estrogens and estrogen-containing oral contraceptives increase TBG concentrations. TBG may also be increased during infectious hepatitis. Decreases in TBG concentrations are observed in nephrosis, acromegaly and after androgen or corticosteroid therapy. Familial hyper- or hypothyroxine-binding globulinemias have been described. The incidence of TBG deficiency approximates 1 in 9000. The binding of thyroxine by thyroxine-binding prealbumin (TBPA) is inhibited by salicylates.
- Medicinal or dietary iodine interferes with all in vivo tests of radioiodine uptake, producing low uptakes which may not be reflective of a true decrease in hormone synthesis.
Carcinogenesis, Mutagenesis, Impairment of Fertility
A reportedly apparent association between prolonged thyroid therapy and breast cancer has not been confirmed and patients on thyroid for established indications should not discontinue therapy. No confirmatory long-term studies in animals have been performed to evaluate carcinogenic potential, mutagenicity, or impairment of fertility in either males or females.
Pregnancy
Pregnancy Category A: Thyroid hormones do not readily cross the placental barrier. The clinical experience to date does not indicate any adverse effect on fetuses when thyroid hormones are administered to pregnant women. On the basis of current knowledge, thyroid replacement therapy to hypothyroid women should not be discontinued during pregnancy.
Nursing Mothers
Minimal amounts of thyroid hormones are excreted in human milk. Thyroid hormones are not associated with serious adverse reactions and do not have a known tumorigenic potential. However, caution should be exercised when thyroid hormones are administered to a nursing woman.
Geriatric Use
Clinical studies of liothyronine sodium did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
ADVERSE REACTIONS
The most frequently reported adverse events were arrhythmia (6% of patients) and tachycardia (3%). Cardiopulmonary arrest, hypotension and myocardial infarction occurred in approximately 2% of patients. The following events occurred in approximately 1% or fewer of patients: angina, congestive heart failure, fever, hypertension, phlebitis and twitching.
In rare instances, allergic skin reactions have been reported with liothyronine sodium tablets.
-
OVERDOSAGE
Signs and Symptoms:Headache, irritability, nervousness, tremor, sweating, increased bowel motility and menstrual irregularities. Angina pectoris, arrhythmia, tachycardia, acute myocardial infarction or congestive heart failure may be induced or aggravated. Shock may also develop if there is untreated pituitary or adrenocortical failure. Massive overdosage may result in symptoms resembling thyroid storm.
Treatment of Overdosage:Dosage should be reduced or therapy temporarily discontinued if signs and symptoms of overdosage appear. Treatment may be reinstituted at a lower dosage. In normal individuals, normal hypothalamic-pituitary- thyroid axis function is restored in six to eight weeks after cessation of therapy following thyroid suppression.
Treatment is symptomatic and supportive. Oxygen may be administered and ventilation maintained. Cardiac glycosides may be indicated if congestive heart failure develops. Beta-adrenergic antagonists have been used advantageously in the treatment of increased sympathetic activity. Measures to control fever, hypoglycemia or fluid loss should be instituted if needed.
-
DOSAGE AND ADMINISTRATION
Adults
Myxedema coma is usually precipitated in the hypothyroid patient of long standing by intercurrent illness or drugs such as sedatives and anesthetics and should be considered a medical emergency. Therapy should be directed at the correction of electrolyte disturbances, possible infection, or other intercurrent illness in addition to the administration of intravenous liothyronine (T 3). Simultaneous glucocorticosteroids are required.Liothyronine sodium injection (T 3) is for intravenous administration only. It should not be given intramuscularly or subcutaneously.
Prompt administration of an adequate dose of intravenous liothyronine (T 3) is important in determining clinical outcome.
Initial and subsequent doses of liothyronine sodium injection (T 3) should be based on continuous monitoring of the patient’s clinical status and response to therapy.
Liothyronine sodium injection (T 3) should normally be administered at least four hours-and not more than 12 hours-apart.
Administration of at least 65 mcg/day of intravenous liothyronine (T 3) in the initial days of therapy was associated with lower mortality.
There is limited clinical experience with intravenous liothyronine (T 3) at total daily doses exceeding 100 mcg/day.No controlled clinical studies have been done with liothyronine sodium injection (T 3). The following dosing guidelines have been derived from data analysis of myxedema coma/precoma case reports collected by SmithKline Beecham Pharmaceuticals since 1963 and from scientific literature since 1956.
An initial intravenous liothyronine sodium injection (T 3) dose ranging from 25 mcg to 50 mcg is recommended in the emergency treatment of myxedema coma/precoma in adults. In patients with known or suspected cardiovascular disease, an initial dose of 10 mcg to 20 mcg is suggested (see WARNINGS). However, both the initial dose and subsequent doses should be determined on the basis of continuous monitoring of the patient’s clinical condition and response to liothyronine sodium injection (T 3) therapy. Normally at least four hours should be allowed between doses to adequately assess therapeutic response and no more than 12 hours should elapse between doses to avoid fluctuations in hormone levels. Caution should be exercised in adjusting the dose due to the potential of large changes to precipitate adverse cardiovascular events. Review of the myxedema case reports indicates decreased mortality in patients receiving at least 65 mcg/day in the initial days of treatment. However, there is limited clinical experience at total daily doses above 100 mcg. See PRECAUTIONS-Drug Interactions for potential interactions between thyroid hormones and digitalis and vasopressors.
Pediatric Use
There is limited experience with liothyronine sodium injection (T 3) in the pediatric population. Safety and effectiveness in pediatric patients have not been established.Switching to Oral Therapy
Oral therapy should be resumed as soon as the clinical situation has been stabilized and the patient is able to take oral medication. When switching a patient to liothyronine sodium tablets from liothyronine sodium injection (T 3), discontinue liothyronine sodium injection (T 3), initiate oral therapy at a low dosage, and increase gradually according to the patient’s response.If L-thyroxine rather then liothyronine sodium is used in initiating oral therapy, the physician should bear in mind that there is a delay of several days in the onset of L-thyroxine activity and that intravenous therapy should be discontinued gradually.
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIOTHYRONINE SODIUM
liothyronine sodium injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:39822-0151 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIOTHYRONINE SODIUM (UNII: GCA9VV7D2N) (LIOTHYRONINE - UNII:06LU7C9H1V) LIOTHYRONINE 10 ug in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) AMMONIA (UNII: 5138Q19F1X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:39822-0151-1 1 in 1 CARTON 08/17/2005 1 1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076923 08/17/2005 Labeler - XGen Pharmaceuticals DJB, Inc. (117380305) Registrant - XGen Pharmaceuticals DJB, Inc. (117380305)