Label: TDVAX- tetanus and diphtheria toxoids adsorbed injection
- NDC Code(s): 14362-0111-3, 14362-0111-4
- Packager: MassBiologics
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated February 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
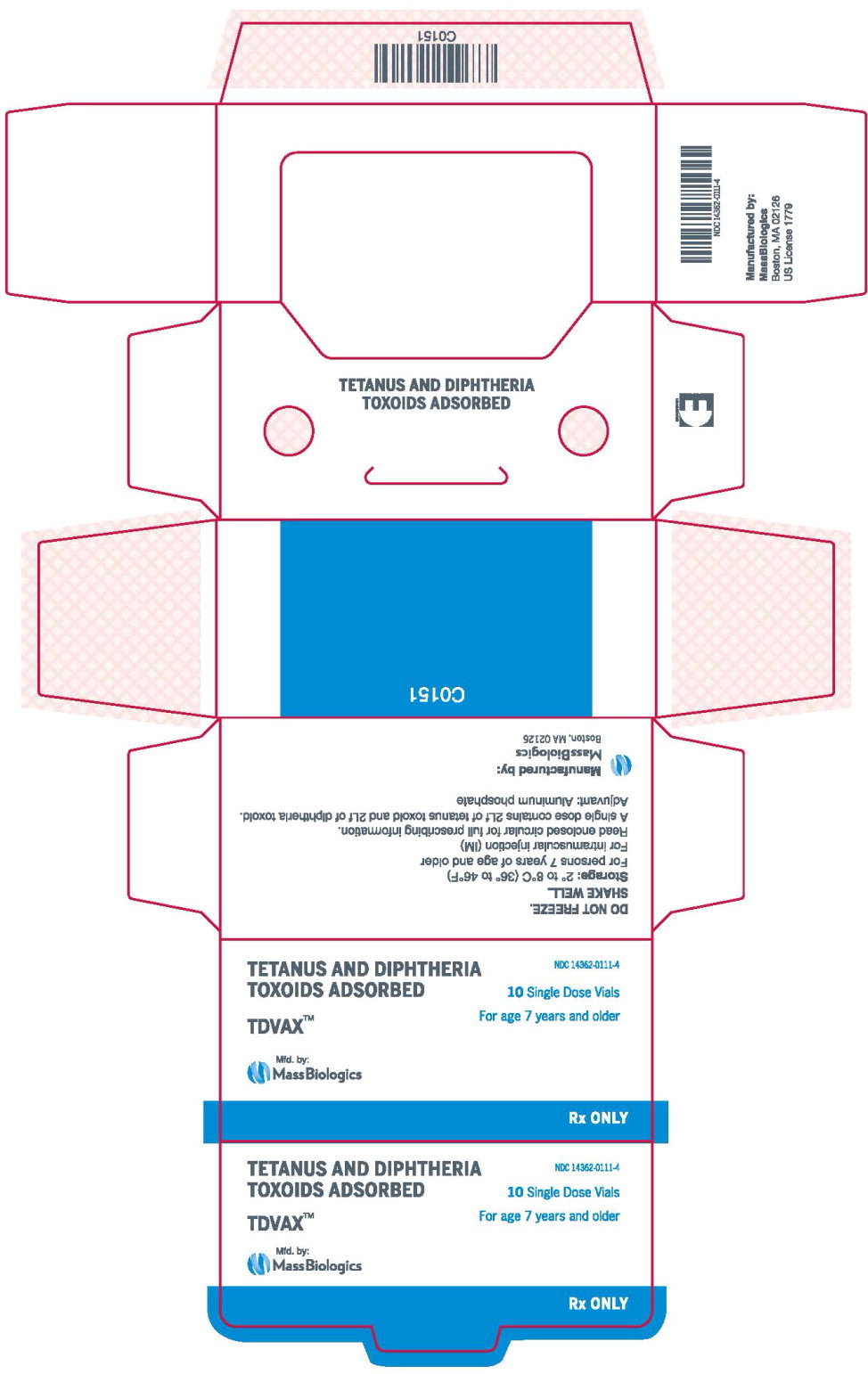
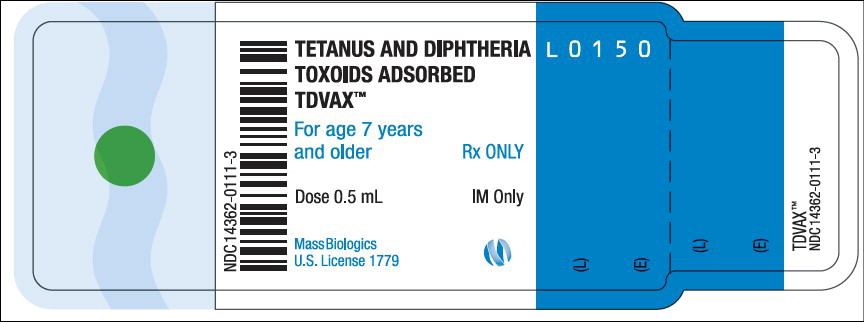
TDVAX ™manufactured by MassBiologics is a sterile vaccine for intramuscular injection. After shaking, the vaccine appears as a homogeneous milky white suspension.
Each 0.5 mL dose of MassBiologics' TDVAX is formulated to contain the following active ingredients: 2 Lf of tetanus toxoid and 2 Lf of diphtheria toxoid. Each 0.5 mL dose also contains aluminum adjuvant (not more than 0.53 mg aluminum by assay), < 100 mcg (0.02%) of residual formaldehyde, and a trace amount of thimerosal [mercury derivative, (≤ 0.3 mcg mercury/dose)] (not as a preservative) from the manufacturing process.
The Corynebacterium diphtheriaeand Clostridium tetaniorganisms are grown on modified Mueller's media 1, 2which contains bovine extracts. The bovine material used in these extracts is sourced from countries which the United States Department of Agriculture has determined neither have nor present an undue risk for bovine spongiform encephalopathy. Tetanus and diphtheria toxins produced during growth of the cultures are detoxified with formaldehyde. The detoxified materials are then separately purified by ammonium sulfate fractionation. The diphtheria toxoid is further purified by column chromatography. The tetanus and diphtheria toxoids are individually adsorbed onto aluminum phosphate.
The tetanus and diphtheria toxoids induce at least 2 units and 1 unit of antitoxin per mL of serum, respectively, in the guinea pig potency test.
-
CLINICAL PHARMACOLOGY
TETANUS
Tetanus (also known as lockjaw) is a serious, often fatal disease caused by an extremely potent neurotoxin produced by C. tetani.
Protection against disease is due to the development of neutralizing antibodies to tetanus toxin. A serum tetanus antitoxin level of 0.01 IU/mL measured via a neutralization assay is considered the minimum protective level. 3, 4The efficacy against tetanus of MassBiologics' TDVAX is supported by the following:
- Response to primary series. Of 20 adults with less than 0.0025 units/mL of tetanus antitoxin in preimmunization serum, 14 (70%) had antitoxin concentrations of 0.01 or greater after 2 doses of TDVAX (2 Lf tetanus toxoid dose). After 3 doses of TDVAX, 16 of 16 adults achieved 0.01 antitoxin units/mL.
- Response to booster doses. Booster doses of TDVAX at doses of 1 Lf and 5 Lf of tetanus toxoidinduced tetanus antitoxin levels greater than 0.01 units/mL when administered to all 36 adults who had received prior tetanus immunizations. 5
DIPHTHERIA
Diphtheria is an acute toxin-mediated disease caused by toxigenic strains of C. diphtheriae. Protection against disease is due to the development of neutralizing antibodies to diphtheria toxin. A serum diphtheria antitoxin level of 0.01 IU/mL is the lowest level giving some degree of protection. 3, 6The efficacy against diphtheria of MassBiologics' TDVAX is supported by the following:
- Response to primary series. Of 10 adults with less than 0.001 units/mL of diphtheria antitoxin in preimmunization serum, 50% had antitoxin concentrations of 0.01 or greater after 2 doses of TDVAX (2 Lf diphtheria toxoid dose). After 3 doses of TDVAX, 6 of 6 adults achieved 0.01 antitoxin units/mL.
- Response to booster doses. In clinical trials, booster doses of TDVAX formulated to contain 1 Lf and 5 Lf of diphtheria toxoid, both induced antitoxin levels greater than 0.01 units/mL when administered to adults with prior diphtheria immunity. 5, 7, 8In 140 adolescent males given a single booster dose of the 1 Lf formulation, all achieved an antitoxin titer of 0.01 units/mL or higher. 8
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
A severe allergic reaction (e.g., anaphylaxis) occurring after a previous dose of this vaccine, or any other tetanus or diphtheria toxoid-containing vaccine, or any component of this vaccine is a contraindication to administration of MassBiologics' TDVAX vaccine. (See DESCRIPTION). Because of the uncertainty as to which component of the vaccine might be responsible, no further vaccination with diphtheria or tetanus components should be carried out. Alternatively, such individuals may be referred to an allergist for evaluation if further immunizations are to be considered.
-
WARNINGS
FREQUENCY OF ADMINISTRATION
More frequent administration of MassBiologics' TDVAX than described in DOSAGE AND ADMINISTRATIONmay be associated with an increased incidence and severity of adverse reactions.
ARTHUS REACTIONS
Persons who experienced an Arthus-type hypersensitivity reaction following a prior dose of a tetanus toxoid-containing vaccine usually have high serum tetanus antitoxin levels and should not receive MassBiologics' TDVAX more frequently than every 10 years, even for tetanus prophylaxis as part of wound management. (See DOSAGE AND ADMINISTRATION).
GUILLAIN-BARRÉ SYNDROME
A review by the Institute of Medicine found evidence for a causal relation between tetanus toxoid and Guillain-Barré Syndrome. 9If Guillain-Barré Syndrome occurred within 6 weeks after receipt of a previous dose of tetanus toxoid-containing vaccine, the decision to give subsequent doses of MassBiologics' TDVAX or any vaccine containing tetanus toxoid should be based on careful consideration of the potential benefits and possible risks. 10
Vaccination with MassBiologics' TDVAX may not protect all individuals.
-
PRECAUTIONS
GENERAL
Epinephrine injection (1:1000) and other appropriate agents and equipment must be immediately available should an acute anaphylactic reaction occur.
Prior to the administration of MassBiologics' TDVAX, the vaccine recipient's current health status and health history should be reviewed. This includes a review of the immunization history of the patient, the presence of any contraindications to immunization, and any adverse events after previous immunizations to allow an assessment of the benefits and risks of vaccination. (See CONTRAINDICATIONSand WARNINGS).
If MassBiologics' TDVAX is administered to immunocompromised persons (whether from disease or treatment) the expected immune response may not be obtained.
INFORMATION FOR PATIENTS
Prior to administration of MassBiologics' TDVAX, patients, parents or guardians should be informed by the health care provider of the benefits and risks of immunization with TDVAX and of the importance of completing the primary immunization series or receiving recommended booster doses.
The health care provider should inform the patient, parent, or guardian of the potential for adverse reactions that have been temporally associated with MassBiologics' TDVAX or other vaccines containing similar ingredients. Patients, parents or guardians should be instructed to report any suspected adverse reactions to their health care provider.
According to the National Childhood Vaccine Injury Act of 1986, Vaccine Information Statements must be provided by the health care provider with each vaccine dose administered. 10
DRUG INTERACTIONS
Patients who are on immunosuppressive therapy, including alkylating agents, antimetabolites, cytotoxic drugs, irradiation, or corticosteroids (used in greater than physiologic doses), may have a reduced immune response to vaccines.
No safety and immunogenicity data are available on the concomitant administration of MassBiologics' TDVAX vaccine with other U.S. licensed vaccines.
CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
No studies have been performed with MassBiologics' TDVAX to evaluate carcinogenicity, mutagenic potential, or impairment of fertility.
PREGNANCY
Animal reproduction studies have not been conducted with MassBiologics' TDVAX. It is also not known whether MassBiologics' TDVAX can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. MassBiologics' TDVAX should be given to a pregnant woman only if clearly needed.
NURSING MOTHERS
It is not known whether MassBiologics' TDVAX is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when MassBiologics' TDVAX is administered to a nursing woman.
-
ADVERSE REACTIONS
DATA FROM CLINICAL TRIALS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine, and may not reflect the rates observed in practice. However, the adverse reaction information from clinical trials provides a basis for identifying adverse events that appear to be related to vaccine use and for approximating rates. Data on adverse reactions following fluid and adsorbed preparations of MassBiologics' TDVAX with various doses of the diphtheria and tetanus components have been reported in a series of studies. 5, 7, 8, 11, 12
POSTMARKETING REPORTS
The following adverse events have been identified during post-approval use of MassBiologics' TDVAX. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequencies or to establish a causal relationship to vaccination. The following adverse events were included because of seriousness or frequency of reporting:
General Disorders and Administration Site Conditions:Injection site reactions, including pain, tenderness, erythema, induration, pruritis, swelling and warmth; peripheral oedema, pyrexia, malaise
Nervous System Disorders:Dizziness, headache, convulsions
Musculoskeletal and Connective Tissue Disorders:Myalgia, musculoskeletal stiffness or pain, arthralgia
Skin and Subcutaneous Tissue Disorders:Rash
Gastrointestinal Disorders:Nausea
Infections and Infestations:Cellulitis
-
DOSAGE AND ADMINISTRATION
PRIMARY IMMUNIZATION
MassBiologics' TDVAX may be used in persons 7 years of age and older who have not been previously immunized against tetanus and diphtheria, as a primary immunization series consisting of three 0.5 mL doses. The first two doses are administered 4-8 weeks apart and the third dose is administered 6-12 months after the second dose.
MassBiologics' TDVAX may be used to complete the primary immunization series for tetanus and diphtheria, following one or two doses of Diphtheria and Tetanus Toxoids and Pertussis Vaccine Adsorbed (wholecell DTP), Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed (DTaP) and/or Diphtheria and Tetanus Toxoids Adsorbed (DT) vaccine. However, the safety and efficacy of MassBiologics' TDVAX in such regimens have not been evaluated.
ROUTINE BOOSTER IMMUNIZATION
MassBiologics' TDVAX may be used for routine booster immunization against tetanus and diphtheria in persons 7 years of age and older who have completed primary immunization against tetanus and diphtheria. Routine booster immunization against tetanus and diphtheria is recommended in children 11-12 years of age and every 10 years thereafter. 10
The Advisory Committee on Immunization Practices (ACIP) has specific recommendations on booster immunization against tetanus and diphtheria for adolescents and adults. 10, 13, 14
TETANUS PROPHYLAXIS IN WOUND MANAGEMENT
For active tetanus immunization in wound management of patients 7 years of age and older, a preparation containing tetanus and diphtheria toxoids is preferred instead of single-antigen tetanus toxoid to enhance diphtheria protection. 15MassBiologics' TDVAX is approved for wound management of patients 7 years of age and older.
The need for active immunization with a tetanus toxoid-containing preparation, with or without Tetanus Immune Globulin (TIG) (Human) depends on both the condition of the wound and the patient's vaccination history ( Table 1).
When indicated, TIG (Human) should be administered using a separate needle and syringe at a different anatomic site, according to the manufacturer's package insert. If a contraindication to using a tetanus toxoid-containing vaccine exists in a person who has not completed tetanus primary immunization and other than a clean, minor wound is sustained, only passive immunization with TIG (Human) should be given. 15
TABLE 1 GUIDE TO TETANUS PROPHYLAXIS IN ROUTINE WOUND MANAGEMENT IN PERSONS AGED 7 YEARS AND OLDER 13, 14, 15 * Such as, but not limited to, wounds contaminated with dirt, feces, soil, and saliva; puncture wounds; avulsions; and wounds resulting from missiles, crushing, burns and frostbite.
† The ACIP has specific recommendations on use of Td or Tetanus Toxoid, Reduced Diphtheria Toxoids and Acellular Pertussis Vaccine Adsorbed (Tdap) in adolescents and adults. 13, 14
If only three doses of fluid tetanus toxoid have been received, then a fourth dose of toxoid, preferablyan adsorbed toxoid, should be given.
Yes, if ≥ 10 years since the last tetanus toxoid-containing vaccine dose.
¶ Yes, if ≥ 5 years since the last tetanus toxoid-containing vaccine dose. (More frequent boosters are not needed and can accentuate side effects.)
History of Adsorbed Tetanus Toxoid (Doses) Clean, Minor Wounds All Other Wounds* Td † TIG Td † TIG Unknown or < 3 Yes No Yes Yes ≥ 3 ‡ No§ No No¶ No DIPHTHERIA PROPHYLAXIS FOR CASE CONTACTS
MassBiologics' TDVAX may be used for post-exposure diphtheria prophylaxis in persons 7 years of age and older who have not completed primary vaccination, whose vaccination status is unknown, or who have not been vaccinated with diphtheria toxoid within the previous 5 years. Consult ACIP recommendations for additional interventions for post-exposure diphtheria prophylaxis. 15
ADMINISTRATION
Shake the vial well to resuspend the vaccine before withdrawing the dose. After shaking, MassBiologics' TDVAX is a homogenous milky white suspension. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If these conditions exist, MassBiologics' TDVAX should not be administered.
Inject 0.5 mL of MassBiologics' TDVAX intramuscularly. The preferred site is the deltoid muscle. The vaccine should not be injected into the gluteal area or areas where there may be a major nerve trunk.
Do not administer this vaccine intravenously, subcutaneously, or intradermally.
MassBiologics' TDVAX should not be combined through reconstitution or mixed with any other vaccine.
- HOW SUPPLIED
-
REFERENCES
- Latham WC, Bent DF, and Levine L. Tetanus toxin production in the absence of protein. Appl. Microbiol. 10(2): March 1962;146-152.
- Mueller JH and Miller PA. Production of diphtheric toxin of high potency (100 Lf) on a reproduciblemedium. J. Immunol. 40: 1941;21-32.
- Food and Drug Administration. Department of Health and Human Services (DHHS). Biological Products; bacterial vaccines and toxoids; implementation of efficacy review; proposed rule. Fed Reg 1985;50(240): 51002-117.
- Wassilak SGF, et al. Tetanus toxoid. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5 thed. Philadelphia, PA: WB Saunders Company; 2008. p. 805-839.
- Ipsen J. Jr. Immunization of adults against diphtheria and tetanus. NEJM. 1954; 251:459-466
- Vitek CR and Wharton M. Diphtheria toxoid. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines.5 thed. Philadelphia, PA: WB Saunders Company; 2008. p. 139-156.
- Edsall G, Altman JS, and Gaspar AJ. Combined tetanus-diphtheria immunization of adults: Use ofsmall doses of diphtheria toxoid. Am J Public Health Nations Health. 1954 Dec;44(12):1537-1545.
- Levine L, Ipsen J, and McComb JA. Adult immunization: preparation and evaluation of combined fluidtetanus and diphtheria toxoids for adult use. Am. J. Hyg. 1961;73:20-35.
- Institute of Medicine. Stratton KR, et al, eds. Adverse Events Associated with Childhood Vaccines: Evidence Bearing on Casuality. Washington D.C.: National Academy Press. 1994:67-117.
- Centers for Disease Control and Prevention. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2006;55 (No. RR-15):1-48.
- McComb JA and Levine L. Adult immunization II. Dosage reduction as a solution to increasing reactions to tetanus toxoid. NEJM. 1961;256:1152-1158.
- Levine L and Edsall G. Tetanus toxoid: What determines reaction proneness? J. Infect. Dis. 1981;144:376.
- Centers for Disease Control and Prevention. Preventing tetanus, diphtheria, and pertussis amongadolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2006;55(No. RR-3):1-43.
- Centers for Disease Control and Prevention. Preventing tetanus, diphtheria, and pertussis amongadults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR 2006;55(No. RR-17):1-37.
- Centers for Disease Control and Prevention. Recommendations of the Immunization Practices Advisory Committee (ACIP). Diphtheria, tetanus, and pertussis: recommendations for vaccine use and other preventive measures. MMWR 1991;40(No. RR-10):1-35.
Manufactured by:
MassBiologics
University of Massachusetts Medical School
Boston, MA 02126
US Government License No. 1779MassBiologics
Product information as of September 2018
- Principal Display Panel – 0.5 mL Carton Label
- Principal Display Panel – 0.5 mL Vial Label
-
INGREDIENTS AND APPEARANCE
TDVAX
tetanus and diphtheria toxoids adsorbed injectionProduct Information Product Type VACCINE Item Code (Source) NDC:14362-0111 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLOSTRIDIUM TETANI TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: K3W1N8YP13) (CLOSTRIDIUM TETANI TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:K3W1N8YP13) CLOSTRIDIUM TETANI TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) 2 [Lf] in 0.5 mL CORYNEBACTERIUM DIPHTHERIAE TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: IRH51QN26H) (CORYNEBACTERIUM DIPHTHERIAE TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:IRH51QN26H) CORYNEBACTERIUM DIPHTHERIAE TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) 2 [Lf] in 0.5 mL Inactive Ingredients Ingredient Name Strength ALUMINUM PHOSPHATE (UNII: F92V3S521O) 0.53 mg in 0.5 mL FORMALDEHYDE (UNII: 1HG84L3525) 100 ug in 0.5 mL THIMEROSAL (UNII: 2225PI3MOV) 0.5 ug in 0.5 mL Product Characteristics Color white (white (after shaking, the vaccine appears as a homogenous milky white suspension) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14362-0111-4 10 in 1 CARTON 1 NDC:14362-0111-3 0.5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101322 07/27/1970 03/31/2025 Labeler - MassBiologics (038051228) Establishment Name Address ID/FEI Business Operations MassBiologics 038051228 manufacture