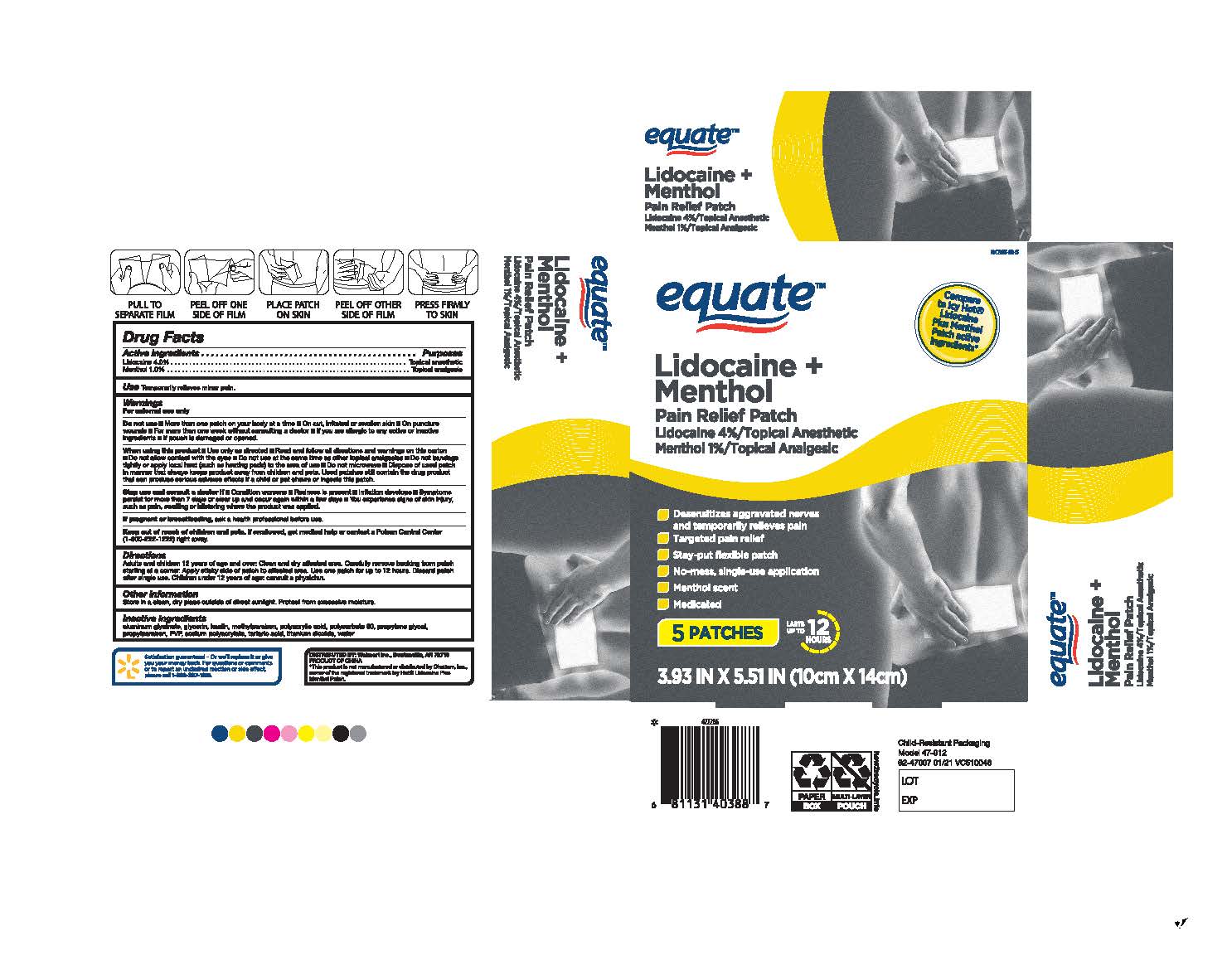
Label: EQUATE LIDOCAINE COOL AND HEAT- lidocaine 4% plus menthol 1% patch
- NDC Code(s): 79903-038-05
- Packager: Walmart Stores Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 25, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- Uses
-
- Warnings
For external use only.
Use only as directed
Read and follow all directions and warnings on this carton
Do not allow contact with the eyes
Do not use at the same time as other topical analgesics
Do not bandage tightly or apply local heat (such as heating pads) to the area of use
Do not microwave
Dispose of the used patch in a manner that always keeps product away from children and pets. Used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch.More than one patch on your body at a time.
On cut, irritated or swollen skin
On puncture wounds
For more than one week without consulting a doctor
If you are allergic to any active or inactive ingredients
If pouch is damaged or opened -
Directions
Adults and children 12 years of age and over: Clean and the dry affected area. Carefully remove backing from the patch starting at a corner. Apply the sticky side of patch to the affected area. Use one patch for up to 12 hours. Discard patch after a single use. Children under 12 years old of age: consult a physician.
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- Equate Lidocaine + Menthol Pain Relief Patch
-
INGREDIENTS AND APPEARANCE
EQUATE LIDOCAINE COOL AND HEAT
lidocaine 4% plus menthol 1% patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79903-038 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 100 g LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) POLYACRYLIC ACID (250000 MW) (UNII: 9G2MAD7J6W) DIHYDROXYALUMINUM AMINOACETATE ANHYDROUS (UNII: 1K713C615K) KAOLIN (UNII: 24H4NWX5CO) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) TARTARIC ACID (UNII: W4888I119H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79903-038-05 5 in 1 BOX 01/01/2021 1 6 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 01/01/2021 Labeler - Walmart Stores Inc (051957769)