Label: ACETAMINOPHEN, DEXTROMETHORPHAN HBR, PHENYLEPHRINE HCL, DOXYLAMINE SUCCINATE capsule, liquid filled
- NDC Code(s): 55629-016-01, 55629-016-02
- Packager: ONE2ZEE LIMITED LIABILITY COMPANY
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 25, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each capsule)
- Purpose
-
Uses
- temporarily relieves common cold/flu symptoms:
ο nasal congestion
ο sinus congestion & pressure
ο cough due to minor throat & bronchial irritation
ο minor aches & pains
ο headache
ο fever
ο sore throat- reduces swelling of nasal passages
- temporarily restores freer breathing through the nose
- promotes nasal and/or sinus drainage
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive.
-
Warning
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take
- more than 10 softgels in 24 hours, which is the maximum daily amount for this product
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Sore throat warning
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use to sedate children.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have ever had an allergic reaction to this product or any of its ingredients
- in children under 12 years of age
Ask a doctor before use if you have
- liver disease
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- glaucoma
- cough with excessive phlegm (mucus)
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema
When using this product
- do not exceed recommended dosage
- may cause marked drowsiness
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
Stop use and ask a doctor if
- pain, cough, or nasal congestion gets worse or lasts more than 7 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- cough comes back or occurs with rash or headache that lasts.
These could be signs of a serious condition.
- nervousness, dizziness, or sleeplessness occurs
If pregnant or breast-feeding, ask a health professional before use.
- Direction
- Other Information:
- Inactive ingredients
-
PRINCIPAL DISPLAY PANEL - Shipping Label
Acetaminophen, Dextromethorphan HBr, Phenylephrine HCL, Doxylamine Succinate capsules
Each Softgel Contains:
(Acetaminophen USP 325 mg, Dextromethorphan Hydrobromide USP 10 mg, Phenylephrine Hydrochloride USP 5mg, Doxylamine Succinate 6.25 mg)LOT NO:
DRUM NO:
MFG DATE:
QUANTITY:
NDC NO: 55629-016-
EXP DATE:WARNING:
KEEP OUT OF REACH OF CHILDRENSTORE CONTROLLED ROOM TEMPERATURE OF 59° - 86°F (15° - 30°C)
PROTECT FROM LIGHT, MOISTURE AND FREEZINGTHIS IS A BULK SHIPMENT INTENDED FOR FURTHER PROCESSING ONLY.
CONTENTS SHOULD BE APPROVED, REPACKAGED IMMEDIATELY AND LABELED IN STRICT CONFORMANCE WITH
THE F.D & C.ACT AND REGULATIONS THEREUNDER.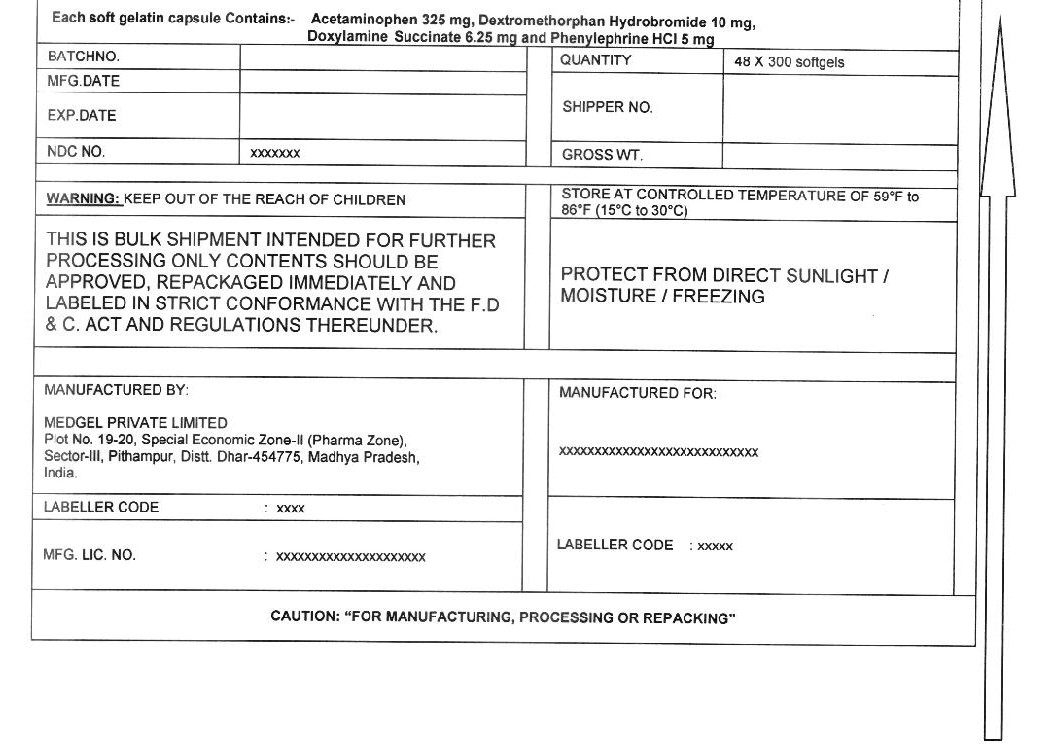
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN, DEXTROMETHORPHAN HBR, PHENYLEPHRINE HCL, DOXYLAMINE SUCCINATE
acetaminophen, dextromethorphan hbr, phenylephrine hcl, doxylamine succinate capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55629-016 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE 5 mg DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 6.25 mg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POVIDONE K30 (UNII: U725QWY32X) GLYCERIN (UNII: PDC6A3C0OX) SODIUM HYDROXIDE (UNII: 55X04QC32I) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GELATIN (UNII: 2G86QN327L) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) Product Characteristics Color green Score no score Shape capsule Size 21mm Flavor Imprint Code IS5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55629-016-01 48 in 1 CARTON 03/02/2021 1 NDC:55629-016-02 300 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 03/02/2021 Labeler - ONE2ZEE LIMITED LIABILITY COMPANY (078656111) Registrant - ONE2ZEE LIMITED LIABILITY COMPANY (078656111) Establishment Name Address ID/FEI Business Operations Medgel Private Limited 677385498 manufacture(55629-016)

