Label: ALLERGY RELIEF- diphenhydramine hcl capsule
- NDC Code(s): 55910-191-08
- Packager: DOLGENCORP, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each banded capsule)
- Purpose
- Uses
-
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
DG™ | health
Compare to
active ingredient
of Benadryl®
Allergy*Allergy Relief
Diphenhydramine HCl, 25 mg
AntihistamineTemporarily Relieves:
• Sneezing • Itchy, watery eyes
• Runny nose • Itchy nose or throatEach capsule individually
banded for your protection24 Capsules
Actual Capsule Size
25
mg eachTAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED
OR IF BLISTER UNIT IS TORN OR BROKEN OR IF
RED BAND AROUND CAPSULE IS BROKEN OR MISSING100%
Satisfaction
Guaranteed!
(888) 309-9030DISTRIBUTED BY
OLD EAST MAIN CO.
100 MISSION RIDGE
GOODLETTSVILLE, TN 37072*This product is not manufactured or distributed by
Johnson & Johnson Corporation, owner of the
registered trademark Benadryl® Allergy.
50844 ORG111719008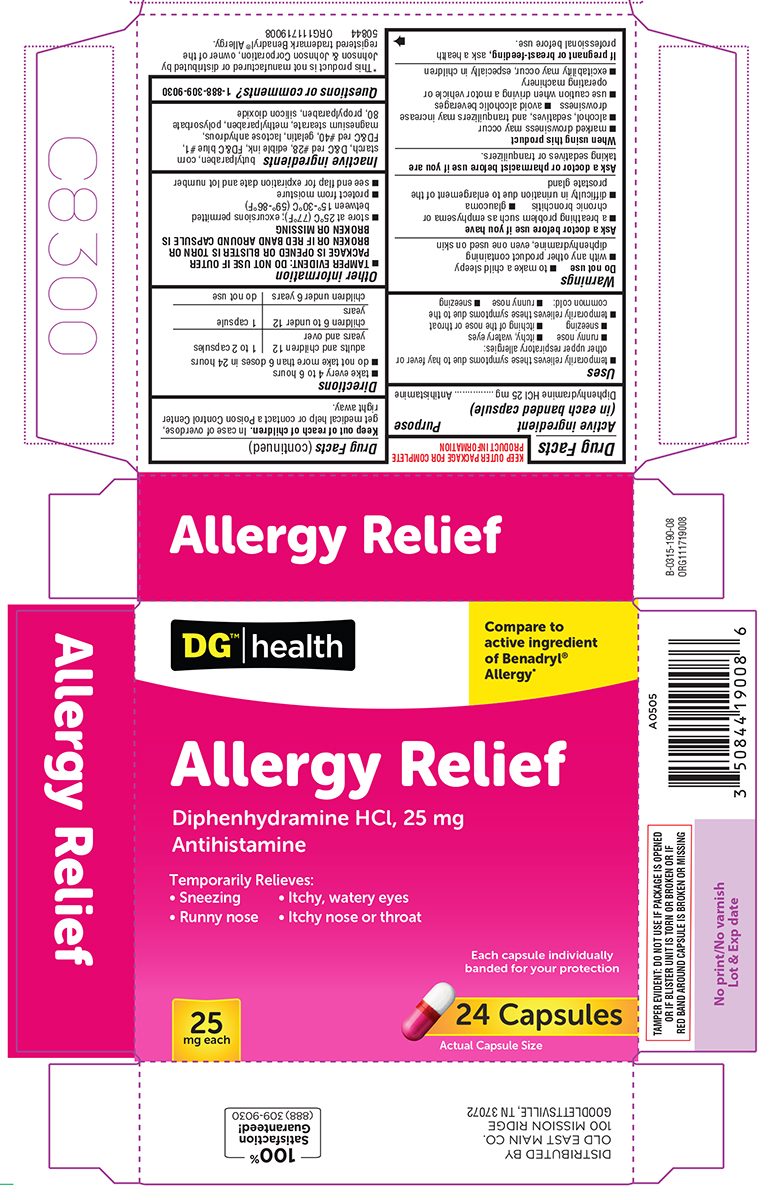
DG Health 44-190
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
diphenhydramine hcl capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55910-191 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength BUTYLPARABEN (UNII: 3QPI1U3FV8) STARCH, CORN (UNII: O8232NY3SJ) D&C RED NO. 28 (UNII: 767IP0Y5NH) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) METHYLPARABEN (UNII: A2I8C7HI9T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLPARABEN (UNII: Z8IX2SC1OH) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color pink, white Score no score Shape CAPSULE Size 14mm Flavor Imprint Code 44;107 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55910-191-08 2 in 1 CARTON 07/30/2020 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 07/30/2020 Labeler - DOLGENCORP, LLC (068331990) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(55910-191) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 pack(55910-191) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 manufacture(55910-191) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(55910-191)

