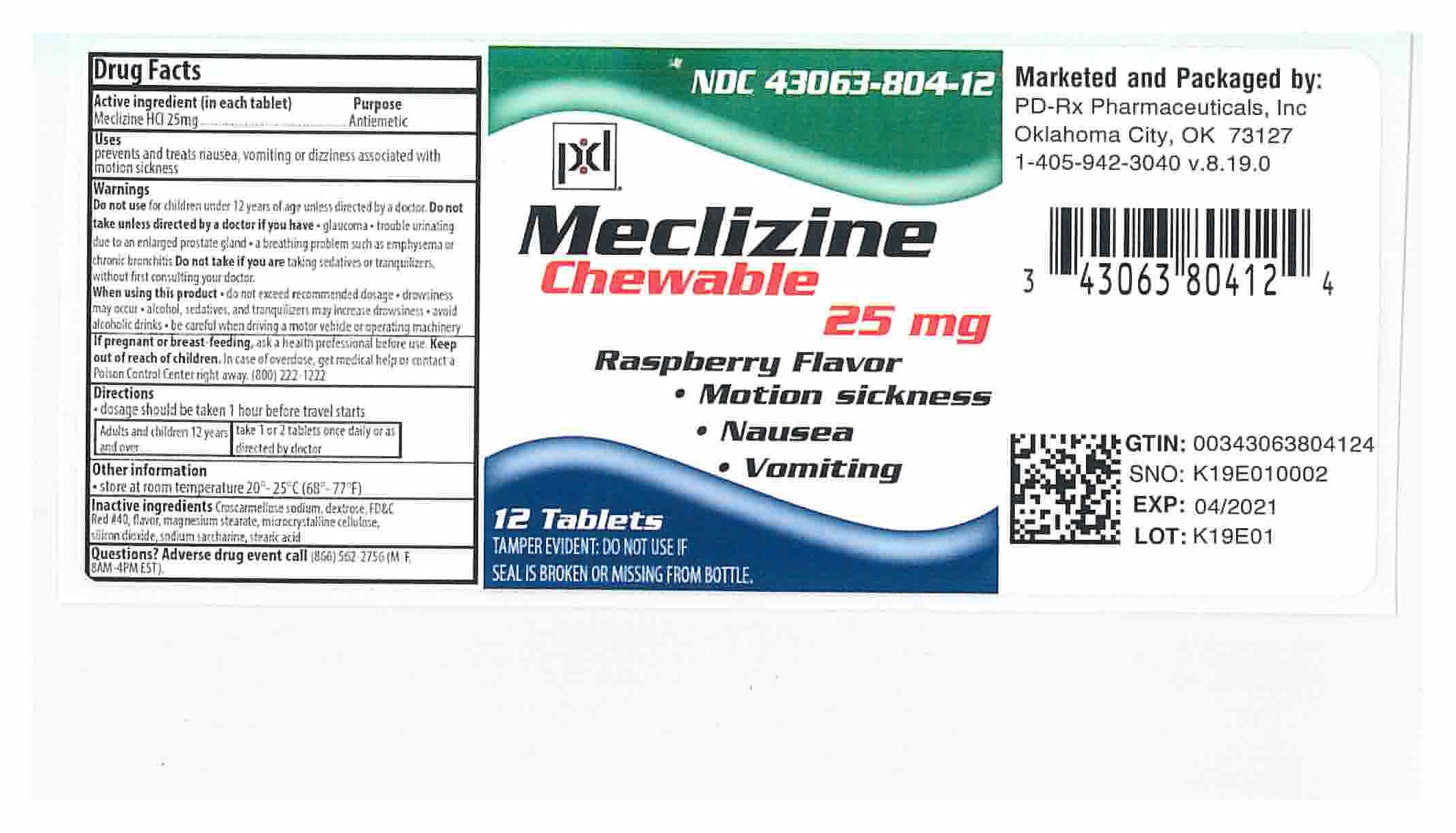
Label: MECLIZINE 25- meclizine hydrochloride tablet, chewable
-
NDC Code(s):
43063-804-01,
43063-804-06,
43063-804-12,
43063-804-20, view more43063-804-30
- Packager: PD-Rx Pharmaceuticals, Inc.
- This is a repackaged label.
- Source NDC Code(s): 16103-387
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients (in each chewable tablet)
- Purpose
- Indications and Usage
-
Warnings
- Do not use for children under 12 years of age unless directed by a doctor.
Do not take unless directed by a doctor if you have
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
- Directions
- Inactive ingredients
- Questions?
- How Supplied
- PRINCIPAL DISPLAY PANEL - 25 mg Bottle Label
-
INGREDIENTS AND APPEARANCE
MECLIZINE 25
meclizine hydrochloride tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43063-804(NDC:16103-387) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MECLIZINE HYDROCHLORIDE (UNII: HDP7W44CIO) (MECLIZINE - UNII:3L5TQ84570) MECLIZINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DEXTROSE (UNII: IY9XDZ35W2) FD&C RED NO. 40 (UNII: WZB9127XOA) SACCHARIN SODIUM (UNII: SB8ZUX40TY) STEARIC ACID (UNII: 4ELV7Z65AP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color pink (LIGHT PINK COLOR) Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code PH051 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43063-804-12 12 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/27/2017 2 NDC:43063-804-20 20 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/12/2018 3 NDC:43063-804-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/08/2018 4 NDC:43063-804-30 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/15/2018 5 NDC:43063-804-06 6 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/23/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M009 11/27/2017 Labeler - PD-Rx Pharmaceuticals, Inc. (156893695) Registrant - PD-Rx Pharmaceuticals, Inc. (156893695) Establishment Name Address ID/FEI Business Operations PD-Rx Pharmaceuticals, Inc. 156893695 repack(43063-804)