Label: AUTO 157- benzalkonium chloride, lidocaine, benzocaine, alcohol, ibuprofen, acetaminophen, aspirin kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 52124-0001-1, 52124-0004-1, 52124-0008-1, 52124-0009-1, view more52124-0010-1, 52124-0011-1, 52124-0118-1 - Packager: Genuine First Aid LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 7, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- DO NOT USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- STOP USE
- DO NOT USE
- WHEN USING
- INACTIVE INGREDIENT
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- STORAGE AND HANDLING
- DO NOT USE
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
Warnings
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include: shock, facial swelling, asthma (wheezing) rash, skin reddening, blisters, hives If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains a nonsteroidal anti-inflammatory drug (NSAID), which may cause severe stomach
bleeding. The chance is higher if you: are age 60 or older, have had stomach ulcers or bleeding problems, take a blood thinner (anticoagulant) or steroid drug, take other drugs containing NSAIDs (aspirin, ibuprofen, naproxen, or others), have 3 or more alcoholic drinks every day while using this product, take more or for a longer time than directed - DO NOT USE
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- WHEN USING
-
STOP USE
Stop use and ask a doctor If:
you experience any of the following signs of stomach bleeding; feel faint; vomit blood; have bloody or black stools; have stomach pain that does get better; pain gets worse or lasts more than 10 days; fever gets worse or lasts more than 3 days; redness or swelling is present in the painful area; any new symptoms appear - PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions:
do not use more than directed; the smallest effective dose should be used; do not take longer than 10 days, unless directed by a doctor.
Adults and Children (12 years and older): Take 1 tablet every 4 to 6 hours while symptoms persist. If pain or fever does not respond to 1 tablet, 2 tablets may be used. Do not exceed 6 tablets in 24 hours, unless directed by a doctor.
Children under 12 years: Do not give to children under 12 years of age. - STORAGE AND HANDLING
- INACTIVE INGREDIENT
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
Warnings:
Liver warning: This product contains acetaminophen.
Severe liver damage may occur if: adult takes more than 12 tablets in 24 hours, which is the maximum daily amount; child takes more than 5 doses in 24 hours; taken with other drugs containing acetaminophen; adult has 3 or more alcoholic drinks every day while using this product - DO NOT USE
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
Adults and Children Take 2 tablets every 4 to 6 hours as
12 years of age needed. Do not take more than 12 tablets
or older in 24 hours.
Children 6-11 years Take 1 tablet every 4 to 6 hours as
of age needed. Do not take more than 5
tablets in 24 hours.
Children under 6 Do not use this regular strength product.
years of age This will provide more than the recommended dose (overdose) and could
cause serious health problems. - STORAGE AND HANDLING
- GENERAL PRECAUTIONS
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox of flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include: hives, skin reddening, facial swelling, rash, asthma (wheezing), blisters, shock, If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
are age 60 or older; have had stomach ulcers or bleeding problems; take a blood thinner (anticoagulant) or steroid drug; take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others); have 3 or more alcoholic drinks every day while using this product; take more or for a longer time than directed - DO NOT USE
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- WHEN USING
-
STOP USE
Stop use and ask a doctor if
you experience any of the following signs of stomach bleeding:
feel faint; vomit blood; have bloody or black stools; have stomach
pain that does not get better; pain gets worse or lasts more than 10 days; fever gets worse or lasts more than 3 days; you have difficulty swallowing; if ringing in the ears or loss of hearing occurs; redness or swelling is present in the painful areas; any new symptoms appear - PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- WHEN USING
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL
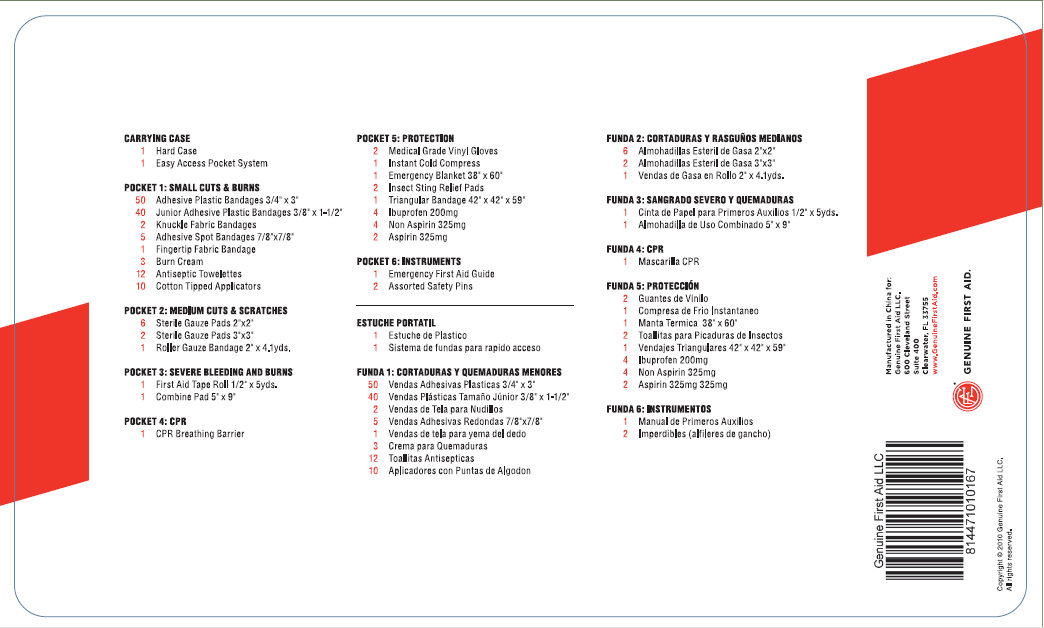
CARRYING CASE
1 Hard Case
1 Easy Access Pocket System
POCKET 1: SMALL CUTS and BURNS
50 Adhesive Plastic Bandages 3/4" X 3"
40 Junior Adhesive Plastic Bandages 3/8" X 1-1/2"
2 Knuckle Fabric Bandages
1 Adhesive Spot Bandages 7/8"X7/8"
1 Fingertip Fabric Bandage
3 Burn Cream
12 Antiseptic Towelettes
10 Cotton Tipped Applicators
POCKET 2: MEDIUM CUTS and SCRATCHES
6 Sterile Gauze Pads 2"X2"
2 Sterile Gauze Pads 3"X3"
1 Roller Gauze Bandage 2" X 4.1yds.
POCKET 3: SEVERE BLEEDING and BURNS
1 First Aid Tape Roll 1/2" X 5yds.
1 Combine Pad 5" X 9"
POCKET 4: CPR
1 CPR Breathing Barrier
POCKET 5: PROTECTION
2 Medical Grade Vinyl Gloves
1 Instant Cold Compress
1 Emergency Blanket 38" X 60"
2 Insect Sting Relief Pads
1 Triangular Bandage 42" X 42" X 59"
4 Ibuprofen 200mg
4 Non Aspirin 325mg
2 Aspirin 325mg
POCKET 6: INSTRUMENTS
1 Emergency First Aid Guide
2 Assorted Safety Pins
-------------------------------------------------------------------------
ESTUCHE PORTATIL
1 Estuche de Plastico
1 Sistema de fundas para rapido acceso
FUNDA 1: CORTADURAS Y QUEMADURAS MENORES
50 Vendas Adhesivas Plasticas 3/4" X 3"
40 Vendas Plasticas Tamano Junior 3/8" X 1-1/2"
2 Vendas de Tela para Nudillos
5 Vendas Adhesivas Redondas 7/8"X7/8"
1 Vendas de tela para yema del dedo
3 Cream para Quemaduras
12 Toallitas Antisepticas
10 Aplicadores con Puntas de Algodon
FUNDA 2: CORTADURAS Y RASGUNOS MEDIANOS
6 Almohadillas Esteril de Gasa 2"X2"
2 Almohadillas Esteril de Gasa 3"X3"
1 Vendas de Gasa en Rollo 2" X 4.1yds.
FUNDA 3: SANGRADO SEVERO Y QUEMADURAS
1 Cinta de papel para Primeros Auxilios 1/2" X 5yds.
1 Almohadilla de Uso Combinado 5" X 9"
FUNDA 4: CPR
1 Mascarilla CPR
FUNDA 5: PROTECCION
2 Guantes de Vinilo
1 Compresa de Frio Instantaneo
1 Manta Termica 38" X 60"
2 Toallitas para Picaduras de Insectos
1 Vendajes Triangulares 42" X 42" X 59"
4 Ibuprofen 200mg
4 Non Aspirin 325mg
2 Aspirin 325mg 325mg
FUNDA 6: INSTRUMENTOS
1 Manual de Primeros Auxilios
2 Imperdibles ( alfileres de gancho )
Genuine First Aid LLC
814471010167
Copyright c 2010 Genuine First Aid LLC.
All rights reserved.
Manufactured in China for:
Genuine First Aid LLC.
600 Cleveland Street
Suite 400
Clearwater, FL 33755
www.GenuineFirstAid.com
GENUINE FIRST AID.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AUTO 157
benzalkonium chloride, lidocaine, benzocaine, alcohol, ibuprofen, acetaminophen, aspirin kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52124-0118 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0118-1 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 3 PACKAGE 2.7 g Part 2 12 PACKAGE 9.6 mL Part 3 2 PACKAGE 1 mL Part 4 2 PACKAGE 4 Part 5 2 PACKAGE 4 Part 6 1 PACKAGE 2 Part 1 of 6 GENUINE FIRST AID BURN ANTISEPTIC PAIN RELIEF WITH ALOE
benzalkonium chloride, lidocaine creamProduct Information Item Code (Source) NDC:52124-0004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 0.5 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0004-1 0.9 g in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part345 05/07/2010 Part 2 of 6 ANTISEPTIC TOWELETTE
benzalkonium chloride liquidProduct Information Item Code (Source) NDC:52124-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.40 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0001-1 0.8 mL in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333 05/08/2010 Part 3 of 6 INSECT STING RELIEF PAD
benzocaine,alcohol liquidProduct Information Item Code (Source) NDC:52124-0008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 6 mL in 100 mL ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 60 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0008-1 0.5 mL in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part348 05/08/2010 Part 4 of 6 IBUPROFEN
ibuprofen tabletProduct Information Item Code (Source) NDC:52124-0009 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength POWDERED CELLULOSE (UNII: SMD1X3XO9M) STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSE (UNII: 3NXW29V3WO) LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color white (White) Score no score Shape ROUND Size 10mm Flavor Imprint Code 44;352 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0009-1 2 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075010 05/08/2010 Part 5 of 6 NON-ASPIRIN
acetaminophen tabletProduct Information Item Code (Source) NDC:52124-0010 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) STEARIC ACID (UNII: 4ELV7Z65AP) POVIDONE (UNII: FZ989GH94E) Product Characteristics Color white (WHITE) Score no score Shape ROUND Size 11mm Flavor Imprint Code AZ;234 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0010-1 2 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part343 05/08/2010 Part 6 of 6 ASPIRIN
aspirin tabletProduct Information Item Code (Source) NDC:52124-0011 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 325 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white (White) Score no score Shape ROUND Size 11mm Flavor Imprint Code 44;157;ASPIRIN Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0011-1 2 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part343 05/08/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333 05/07/2010 Labeler - Genuine First Aid LLC (619609857) Establishment Name Address ID/FEI Business Operations GFA Production ( Xiamen) Co., Ltd 421256261 manufacture