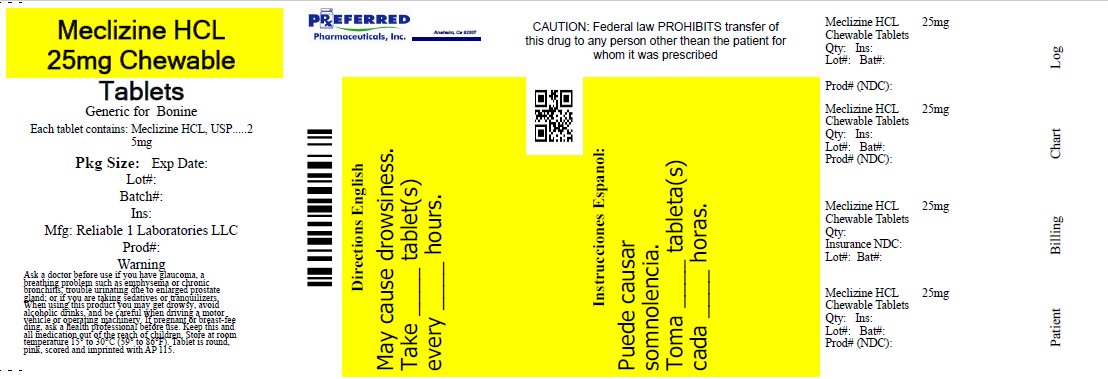
Label: MECLIZINE HCL 25 MG- meclizine hcl tablet
- NDC Code(s): 68788-7982-1, 68788-7982-3
- Packager: Preferred Pharmaceuticals Inc.
- This is a repackaged label.
- Source NDC Code(s): 69618-028
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- •
- glaucoma
- •
- a breathing problem such as emphysema or chronic bronchitis
- •
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers
When using this prodcut
- •
- you may get drowsy
- •
- avoid alcoholic drinks
- •
- alcohol, sedatives and tranquilizers may increase drowsiness
- •
- be careful when driving a motor vehicle or operating machinery
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- WHEN USING
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MECLIZINE HCL 25 MG
meclizine hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68788-7982(NDC:69618-028) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MECLIZINE HYDROCHLORIDE (UNII: HDP7W44CIO) (MECLIZINE - UNII:3L5TQ84570) MECLIZINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) FD&C RED NO. 40 (UNII: WZB9127XOA) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) RASPBERRY (UNII: 4N14V5R27W) ASPARTAME (UNII: Z0H242BBR1) SUCROSE (UNII: C151H8M554) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) Product Characteristics Color pink (Light Raspberry color) Score 2 pieces Shape ROUND Size 8mm Flavor RASPBERRY Imprint Code AP;115 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68788-7982-1 10 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/28/2021 2 NDC:68788-7982-3 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/28/2021 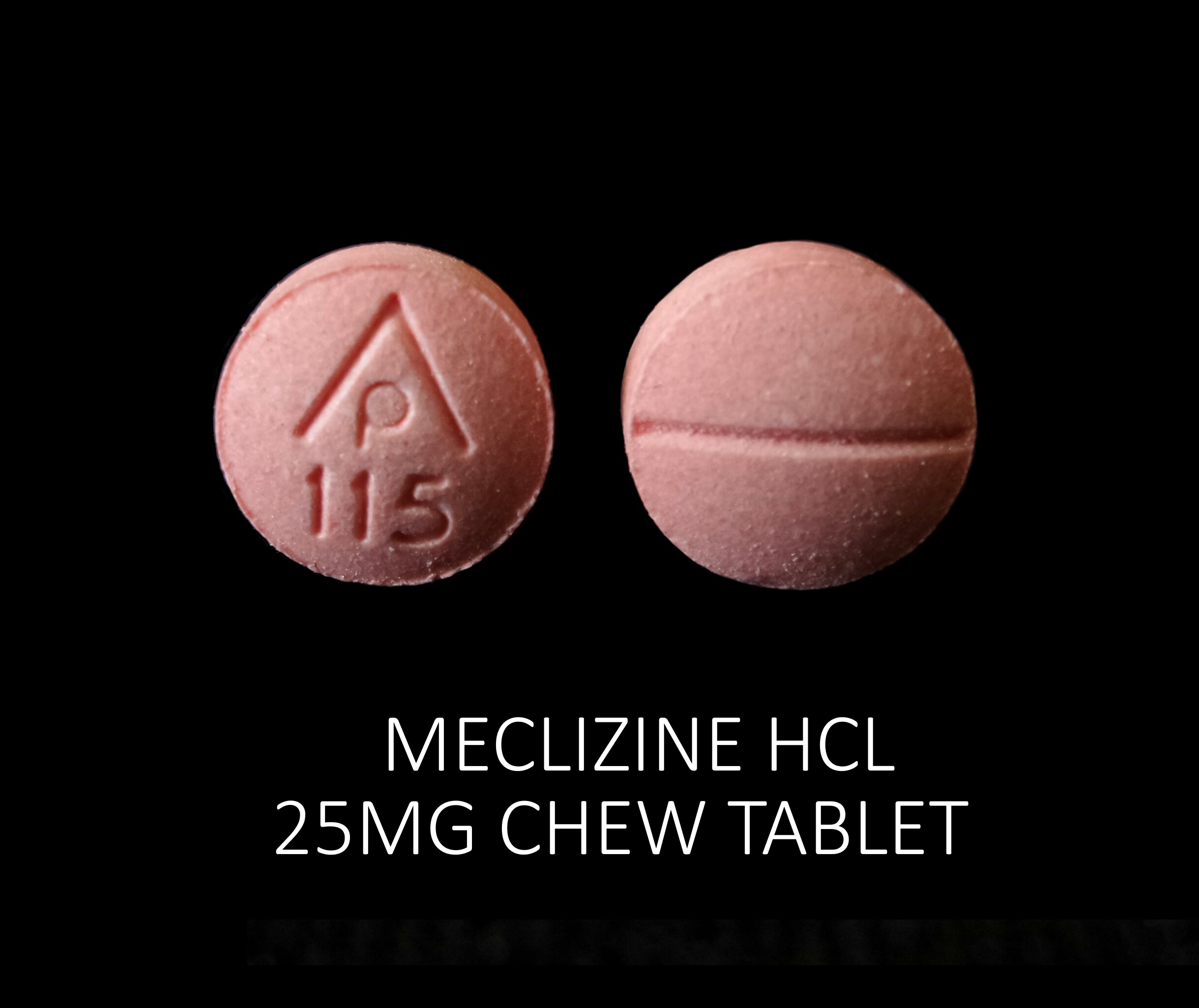
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug part336 07/28/2021 Labeler - Preferred Pharmaceuticals Inc. (791119022) Registrant - Preferred Pharmaceuticals Inc. (791119022) Establishment Name Address ID/FEI Business Operations Preferred Pharmaceuticals Inc. 791119022 REPACK(68788-7982)