Label: BCG VACCINE- bacillus calmette-guerin substrain tice live antigen injection, powder, lyophilized, for solution
- NDC Code(s): 0052-0603-01, 0052-0603-02
- Packager: Merck Sharp & Dohme LLC
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated October 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
BCG VACCINE for percutaneous use is an attenuated, live culture preparation of the Bacillus of Calmette and Guerin (BCG) strain of Mycobacterium bovis.{1} The TICE® strain used in this BCG VACCINE preparation was developed at the University of Illinois from a strain originated at the Pasteur Institute.
The medium in which the TICE® BCG organism is grown for preparation of the freeze-dried cake is composed of the following ingredients: glycerin, asparagine, citric acid, potassium phosphate, magnesium sulfate, and iron ammonium citrate. The final preparation prior to freeze drying also contains lactose. The freeze-dried BCG preparation is delivered in vials, each containing 1 to 8 × 108 colony forming units (CFU) of BCG which is equivalent to approximately 50 mg wet weight. Determination of in-vitro potency is achieved through colony counts derived from a serial dilution assay. Intradermal guinea pig testing is also used as an indirect measure of potency.
Reconstitution requires addition of Sterile Water for Injection, USP at 4-25°C (39-77°F). For an adult dosage, 1 mL of Sterile Water for Injection, USP, should be added to one single-dose vial of vaccine. For a pediatric dosage, 2 mL of Sterile Water for Injection, USP, should be added to one single-dose vial of vaccine (see DOSAGE AND ADMINISTRATION).
No preservatives have been added.
-
CLINICAL PHARMACOLOGY
Tuberculosis (TB) is primarily an airborne communicable disease caused by the bacterium, Mycobacterium tuberculosis.
Tuberculosis is an important global public health problem with an estimated 8–10 million cases and 2–3 million deaths occurring each year.{2} The control of TB in the United States has been a constant challenge particularly with the resurgence in TB in the late 1980s and the early 1990s. In the United States, TB had declined approximately 6% per year since nationwide reporting began in 1953. However, in 1985 there was a 1.1% increase over the previous year. This upward trend continued through 1992, when the incidence was 10.5 cases per 100,000 population. In 1993, there was a 5.2% decrease over 1992 with a rate of 9.8 cases per 100,000 population.{3} In 1997, the total TB cases reported was 19,855 or 7.4 cases per 100,000 people. This incidence rate represented the fifth consecutive year that number of reported TB cases had declined and a 26% decrease since the peak in 1992.{4}
In the 1990s, drug-resistant TB also became a significant public health concern. During the period of 1993–1996, in the United States, 13.1% of TB patients were infected with TB strains that were resistant to at least one drug used as first-line treatment for TB (isoniazid, rifampin, pyrazinamide, ethambutol, and streptomycin) and 2.2% of TB patients were infected with TB strains that were multiple drug resistant (MDR as defined by resistance to both isoniazid and rifampin). Cases of MDR-TB were reported from 42 states and Washington D.C. during this time period.{5}
Most persons infected with M. tuberculosis remain infected for many years by developing latent infections. Active TB will reactivate during the lifetime of 5–15% of infected patients who are immunocompetent. In general, active TB is fatal for about 50% of persons who have not been treated.{3} The greatest known risk factor for developing active TB disease is immunodeficiency, particularly if caused by coinfection with HIV.{4} Persons infected with HIV are estimated to be over one hundred times as likely as uninfected persons to develop TB, primarily as a result of reactivation of a latent TB infection.{6} Other groups at high risk for developing TB include foreign-born individuals and persons in institutional settings such as correctional facilities, shelters for the homeless, and nursing homes.
Although over 2 billion people have been immunized with BCG, and it is currently an officially recommended vaccine in more than 180 countries, excluding the U.S., the efficacy of BCG as a vaccine against tuberculosis remains controversial. Prospective vaccine efficacy trials have shown that the protective benefit of BCG (various strains from different manufacturers) against clinical TB was variable, ranging from 0–80%.{7} A recent meta-analysis of data from 14 prospective trials and 12 case control studies concluded that the overall protective effect of BCG against tuberculosis infection was 50%.{8} The reasons for the wide range of effectiveness seen in these studies are unknown but may be attributed to the following: vaccination was not allocated randomly in observational studies; there were differences in BCG strains, methods, and routes of administration; and there were differences in the characteristics of the populations and environments in which the vaccines were studied.{9} Despite the conflicting results concerning prevention of pulmonary tuberculosis, it is widely acknowledged that immunization of infants with BCG lowers the risk of disseminated complications of this disease. Estimates in areas where BCG vaccination is performed at birth indicate that the effectiveness of BCG in preventing childhood TB meningitis or miliary TB exceeds 70%.{10-15}
In a prospective trial using the TICE® strain of BCG VACCINE, Rosenthal, et al., studied 1,716 vaccinated and 1,665 non-vaccinated infants, all born at the Cook County Hospital in Chicago and followed for 12–23 years. The diagnosis of tuberculosis was made following a review of chest X-ray results and clinical findings. There were 17 cases of tuberculosis among the vaccinated (0.43/1,000/yr) and 65 cases in the nonvaccinated (1.7/1,000/yr); this is a reduction of 75% (p<0.001) in cases of tuberculosis. One death was attributed to tuberculosis in the vaccinated group with 6 deaths in the controls, or a reduction of 83%. There were 639 families in which there was a sibling in both the control and vaccination groups. Eight of the 790 vaccinated subjects developed tuberculosis as compared with 30 of the 945 controls (p<0.001). Thirteen cases of nonfatal tuberculosis developed in the control group that were 2 years of age and under, with none in the vaccinated group.
There were 3 deaths from tuberculosis in the control group that were less than 2.5 years of age (all had miliary tuberculosis with meningitis), with one death in the vaccinated group (meningitis). The infant who died in the vaccinated group had not converted to a positive purified protein derivative (PPD) skin test at 6 months of age and was never subsequently revaccinated. Following a single vaccination, 99.3% of all infants studied became PPD positive, with 84.2% still being positive after 8 years.{16}
In a 1995 study of vaccine potency, 26 tuberculin negative subjects were vaccinated with BCG VACCINE (TICE® strain) and subsequent tuberculin conversion was monitored. Conversion from a negative to a positive skin test may be considered a surrogate indicator of potency and immunization efficacy of BCG Vaccines; however, the correlation between PPD conversion and vaccine effectiveness has not been established. Twenty-four (24) subjects returned for follow-up testing with PPD 10 tuberculin units (10 TU) 8 weeks after vaccination. Twenty-two (22) of the 24 subjects converted to positive (skin test reading >5mm induration at 48 hours) and 2 remained negative. The conversion rate was 92% and the average positive skin test reading was 15.5mm in induration.{17}
In a second study, 22 volunteers between the ages of 18 and 40 who were not health care workers, were not foreign born, were HIV negative, and were negative responders to a 10 TU PPD skin test were vaccinated with the standard dose of BCG VACCINE (TICE® strain). Eight weeks after vaccination the subjects returned for a 10 TU skin test. Twenty-one (21) out of 22 converted to PPD positive at a level greater than 5mm for a skin test conversion rate of 95%.{17}
-
INDICATIONS AND USAGE
BCG VACCINE (TICE® strain) is indicated for the prevention of tuberculosis in persons not previously infected with M. tuberculosis who are at high risk for exposure. As with any vaccine, immunization with BCG VACCINE may not protect 100% of susceptible individuals.
The Advisory Committee on Immunization Practices (ACIP) and the Advisory Committee for the Elimination of Tuberculosis has recommended that BCG vaccination be considered in the following circumstances.{3}
TB Exposed Tuberculin Skin Test-Negative Infants and Children
BCG vaccination is recommended for infants and children with negative tuberculin skin tests who are (a) at high risk of intimate and prolonged exposure to persistently untreated or ineffectively treated patients with infectious pulmonary tuberculosis and who cannot be removed from the source of exposure and cannot be placed on long-term primary preventive therapy, or (b) continuously exposed to persons with infectious pulmonary tuberculosis who have bacilli resistant to isoniazid and rifampin, and the child cannot be separated from the presence of the infectious patient.{3}
TB Exposed Health Care Workers (HCW) in High Risk Settings
BCG vaccination of HCWs should be considered on an individual basis in settings where (a) a high percentage of TB patients are infected with M. tuberculosis strains resistant to both isoniazid and rifampin, (b) transmission of such drug resistant M. tuberculosis strains to HCWs and subsequent infection are likely, and (c) comprehensive TB infection control precautions have been implemented and have not been successful. Vaccination should not be required for employment or for assignment of HCWs in specific work areas. HCWs considered for BCG vaccination should be counseled regarding the risks and benefits associated with both BCG vaccinations and TB preventive therapy.{3}
-
CONTRAINDICATIONS
BCG VACCINE for prevention of tuberculosis should not be given to persons (a) whose immunologic responses are impaired because of HIV infections, congenital immunodeficiency such as chronic granulomatous disease or interferon gamma receptor deficiency, leukemia, lymphoma, or generalized malignancy or (b) whose immunologic responses have been suppressed by steroids, alkylating agents, antimetabolites, or radiation.{3} BCG VACCINE should not be administered to HIV-infected or immunocompromised infants, children, or adults.
Prior to administration, the possibility of allergic reactions should be assessed. Allergy to any component of BCG VACCINE or an anaphylactic or allergic reaction to a previous dose of BCG VACCINE are contraindications for vaccination.
BCG VACCINE is not a vaccine for the treatment of active tuberculosis.
BCG VACCINE should not be used in infants, children, or adults with severe immune deficiency syndromes. Children with a family history of immune deficiency disease should not be vaccinated; if they are, an infectious disease specialist should be consulted and anti-tuberculous therapy administered if clinically indicated.{18}
-
WARNINGS
Administration should be by the percutaneous route with the multiple puncture device as described below. DO NOT INJECT INTRAVENOUSLY, SUBCUTANEOUSLY, INTRAMUSCULARLY, OR INTRADERMALLY.
Although BCG vaccination often results in local adverse effects, serious or long-term complications are rare. Reactions that can be expected after vaccination include moderate axillary or cervical lymphadenopathy and induration and subsequent pustule formation at the injection site; these reactions can persist for as long as 3 months after vaccination. More severe local reactions include ulceration at the vaccination site, regional suppurative lymphadenitis with draining sinuses, and caseous lesions or purulent drainage at the puncture site; these manifestations might occur within the 5 months after vaccination and could persist for several weeks.
Acute, localized irritative toxicities of BCG may be accompanied by systemic manifestations, consistent with a "flu-like" syndrome. Systemic adverse effects of 1–2 days' duration such as fever, anorexia, myalgia, and neuralgia, often reflect hypersensitivity reactions. However, symptoms such as fever of 103°F or greater, or acute localized inflammation persisting longer than 2–3 days suggest active infections, and evaluation for serious infectious complication should be considered. If a BCG infection is suspected, the physician should consult with an infectious disease expert before therapy is initiated. Treatment should be started without delay. In patients who develop persistent fever or experience an acute febrile illness consistent with BCG infection, two or more antimycobacterial agents should be administered while diagnostic evaluation, including cultures, is conducted. Negative cultures do not necessarily rule out infection. Physicians or persons caring for patients that use this product should be familiar with the literature on prevention, diagnosis, and treatment of BCG-related complications and, when appropriate, should consult an infectious disease specialist or other physician with experience in the diagnosis and treatment of mycobacterial infections.
The most serious complication of BCG vaccination is disseminated BCG infection. BCG osteitis affecting the epiphyses of the long bones, particularly the epiphyses of the leg, can occur from 4 months to 2 years after vaccination. Fatal disseminated BCG disease has occurred at a rate of 0.06–1.56 cases per million doses of vaccine administered; these deaths occurred primarily among immunocompromised persons.{3} The appropriate therapy for systemic BCG infections is discussed in the ADVERSE REACTIONS section.
-
PRECAUTIONS
General
BCG VACCINE contains live bacteria and should be used with aseptic technique. To avoid cross-contamination, parenteral drugs should not be prepared in areas where BCG VACCINE has been in use.{19} A separate sterile multiple puncture device must be used for each patient and appropriately discarded after use. All equipment, supplies and receptacles in contact with BCG VACCINE should be handled and disposed of as biohazardous (see DOSAGE AND ADMINISTRATION).
BCG VACCINE administration should not be attempted in individuals with severe immune deficiency disease. BCG VACCINE should be administered with caution to persons in groups at high risk for HIV infection.
A review of each patient's immunization records to include history on reactions to immunizations should be completed prior to vaccination. All precautions should be taken for the prevention of allergic or any other side reactions, including understanding the use of the biological and the nature of the adverse reactions that may follow its use. Epinephrine injection (1:1000) for the control of immediate allergic reactions must be available should an acute anaphylactic reaction occur.
Vaccination is recommended only for those who are tuberculin negative to a recent skin test with 5 TU.
After BCG vaccination, it is usually not possible to clearly distinguish between a tuberculin reaction caused by persistent post-vaccination sensitivity and one caused by a virulent suprainfection. Caution is advised in attributing a positive skin test to BCG vaccination. A sharp rise in the tuberculin reaction since the latest test should be further investigated (except in the immediate post-vaccination period).
Information for Patients
Before administration of BCG VACCINE, health care personnel should inform patients or guardians of the benefits and risks of immunization and inquire about the health status of the patient. Health care workers considering BCG vaccination should be counseled regarding the risks and benefits associated with both BCG vaccination and TB preventive therapy. They should be informed about (a) the variable data concerning the efficacy of BCG vaccination, (b) the interference with the diagnosis of newly acquired M. tuberculosis infections in BCG-vaccinated persons, and (c) the potential serious complications associated with BCG vaccination of immunocompromised individuals. Health care workers should be informed about (a) the lack of data regarding the efficacy of preventive therapy for MDR-TB infections and (b) the risks of drug toxicity associated with multi-drug preventive therapy regimens.
Following BCG vaccination, no dressing is required; however, it is recommended that the site be loosely covered and kept dry for 24 hours. The vaccination site should be kept clean until the local reaction has disappeared. The patient should be advised that the vaccine contains live organisms. Although the vaccine will not survive in a dry state for long, infection of others is possible. Following vaccination with BCG, initial skin lesions usually appear within 10–14 days and consist of small red papules at the vaccination site. The papules reach a maximum diameter (about 3 mm) after 4 to 6 weeks, after which they may scale and slowly subside. Six months afterwards there is usually no visible sign of the vaccination, though on occasion, a faintly discernible pattern of the points from the multiple puncture device may be visible. On individuals whose skin tends to form keloids, there may be slightly more visible evidence of the vaccination. Any unusual adverse reactions should be reported to the health care provider.
Patients may experience "flu-like" symptoms for 24–48 hours following BCG vaccination. However, the patient should consult with their physician immediately if they experience fever of 103°F or greater, or acute local reactions persisting longer than 2–3 days.
Laboratory Tests
BCG vaccination results in tuberculin skin test reactivity. Tuberculin skin test reactivity as a result of BCG vaccination cannot be readily differentiated from reactivity following exposure to tuberculosis. BCG vaccination should not be administered to individuals with a positive tuberculin skin test.
Prior administration of BCG vaccine has not been associated with a positive interferon gamma release assay (IGRA) test, which are indirect tests for M. tuberculosis infection (including disease) and are intended for use in conjunction with risk assessment, radiography and other medical and diagnostic evaluations.{20,21}
Drug Interactions
Antimicrobial or immunosuppressive agents may interfere with the development of the immune response and should be used only under medical supervision.
Since BCG is a live vaccine, the immune response to the vaccine might be impaired if administered within 30 days of another live vaccine. However, no evidence exists for currently available vaccines to support this concern. Whenever possible, live vaccines administered on different days should be administered at least 30 days apart.{22}
Carcinogenesis, Mutagenesis, Impairment of Fertility
BCG VACCINE has not been evaluated for carcinogenic, mutagenic potentials or impairment of fertility.
Pregnancy
Animal reproduction studies have not been conducted with BCG VACCINE. It is also not known whether BCG VACCINE can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Although no harmful effects to the fetus have been associated with BCG VACCINE, its use is not recommended during pregnancy.{3}
Nursing Mothers
It is not known whether BCG is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions from BCG in nursing infants, a decision should be made whether to discontinue nursing or not to vaccinate, taking into account the importance of tuberculosis vaccination to the mother.
Pediatric Use
See Treatment and Schedule under DOSAGE AND ADMINISTRATION section. Precautions should be taken with respect to infants vaccinated with BCG and exposed to persons with active tuberculosis.{23}
Geriatric Use
Clinical studies of BCG VACCINE did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in response between elderly and younger patients. An intact immune system is a prerequisite for BCG vaccination. If the immune status of an elderly patient, or any patient, is in question, the BCG vaccination should be held until the immune status of the patient has been evaluated.
-
ADVERSE REACTIONS
Although BCG vaccination often causes local reactions, serious or long-term complications are rare.{3} Reactions that can be expected after vaccination include moderate axillary or cervical lymphadenopathy and induration and subsequent pustule formation at the injection site; these reactions can persist for as long as 3 months after vaccination. More serious local reactions include ulceration at the vaccination site, regional suppurative lymphadenitis with draining sinuses, and caseous lesions or purulent draining at the puncture site. These manifestations might occur up to 5 months after vaccination and could persist for several weeks. The intensity and duration of the local reaction depends on the depth of penetration of the multiple puncture device and individual variations in patients' tissue reactions. Slight tenderness at the puncture site may be encountered as well as some itching. The initial skin lesions usually appear within 10–14 days and consist of small red papules at the site. The papules reach maximum diameter (about 3 mm) after 4 to 6 weeks, after which they may scale and then slowly subside.
The most serious complication of BCG vaccination is disseminated BCG infection.{24,25} The most frequent disseminated infection is BCG osteomyelitis (0.01 to 43 cases per million doses of vaccine administered) which usually occurs 4 months to 2 years after vaccination. Fatal disseminated BCG infection has occurred at a rate of 0.06–1.56 cases per million doses; these deaths occurred primarily among immunocompromised persons.
BCG Vaccination of Individuals Infected with HIV
The safety of BCG vaccination in HIV-infected adults and children, including infants, has not been determined by controlled or large studies. This is a concern because of the association between disseminated BCG infection and underlying immunosuppression. Individuals with HIV infection should not receive the BCG VACCINE.{3}
Treatment of Adverse Reactions
If a systemic BCG infection occurs, an infectious disease expert should be consulted and anti-tuberculosis therapy should be initiated. Since BCG strains are resistant to pyrazinamide, this antibiotic should not be used.
Reporting of Adverse Reactions
All suspected adverse reactions to BCG vaccination should be reported to Merck Sharp & Dohme LLC, Rahway, NJ USA at 1-877-888-4231 and to the Vaccine Adverse Event Reporting System (VAERS); telephone 1-800-822-7967. These reactions occasionally could occur more than 1 year after vaccination.
-
OVERDOSAGE
Accidental overdosages if treated immediately with anti-tuberculous drugs have not led to complications.{26} If the vaccination response is allowed to progress it can still be treated successfully with anti-tuberculous drugs but complications can include regional adenitis, lupus vulgaris, subcutaneous cold abscesses, ocular lesions, and others.{27}
-
DOSAGE AND ADMINISTRATION
Preparation of Agent
The preparation of the BCG VACCINE suspension should be done using aseptic technique. To avoid cross-contamination, parenteral drugs should not be prepared in areas where BCG VACCINE has been prepared. A separate area for the preparation of the BCG VACCINE suspension is recommended. All equipment, supplies and receptacles in contact with BCG VACCINE should be handled and disposed of as biohazardous. The pharmacist or individual responsible for mixing the agent should wear gloves and take precautions to avoid contact of BCG with broken skin. If preparation cannot be performed in a biocontainment hood, then a mask and gown should be worn to avoid inhalation of BCG organisms and inadvertent exposure to broken skin.
Using aseptic methods, 1 mL of Sterile Water for Injection, USP at 4-25°C (39-77°F), is added to one vial of vaccine (see Pediatric Dose below for pediatric use). Gently swirl the vial until a homogenous suspension is obtained. Avoid forceful agitation which may cause clumping of the mycobacteria.
Persons administering vaccines should take necessary precautions to minimize risk for spreading disease. Hands should be washed before each new patient is seen. Syringes and needles used for applications must be sterile and preferably disposable to minimize the risk of contamination. A separate needle and syringe should be used for each application. Disposable needles and the multiple puncture device should be discarded as biohazardous waste in labeled, puncture-proof containers to prevent inadvertent needlestick injury or reuse.{22} After use, any unused vaccine and all materials exposed to the product should be immediately placed in a biohazard container and disposed of in an appropriate manner.
Reconstituted vaccine should be kept refrigerated, protected from exposure to direct sunlight, and used within 2 hours. Freezing of the reconstituted product is not recommended. Discard unused portion.
Note: DO NOT filter the contents of the BCG VACCINE vial. Precautions should be taken to avoid exposing the BCG VACCINE to direct sunlight. Bacteriostatic solutions must be avoided. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Reconstitution should result in a uniform suspension of the bacilli.
Treatment and Schedule
BCG vaccination is reserved for persons who have a reaction of less than 5mm induration after skin testing with 5 TU of PPD tuberculin. The preferred method of skin testing is the Mantoux tuberculin skin-test using 0.1 mL of 5 tuberculin units (TU) of PPD.{3} It is recommended that a Mantoux skin-test be performed prior to BCG vaccination to demonstrate the absence of tuberculous infection.
The vaccine is to be administered after fully explaining the risks and benefits to the vaccinee, parent, or guardian. BCG vaccination should not be given to individuals previously infected with M. tuberculosis. The vaccine is administered percutaneously utilizing a sterile, single-use multiple puncture device. The multiple puncture device consists of a plastic holder for a thin, wafer-like stainless steel plate from which 36 points protrude (Figure 1). After the vaccine is prepared, the skin site is cleansed with an alcohol or acetone sponge and allowed to dry thoroughly.
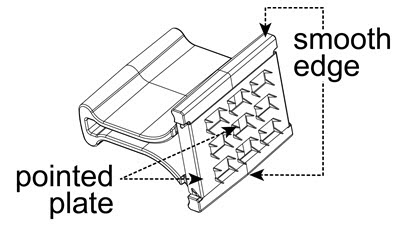
Figure 1
- 1.
- Administer the vaccine in the deltoid region (Figure 2). Position the arm to maintain a horizontal surface where the vaccine is to be placed.
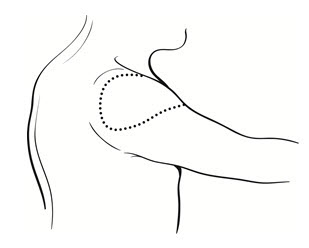
Figure 2
- 2.
- Drop the immunizing dose of 0.2–0.3 mL of BCG VACCINE from the syringe and needle onto the cleansed surface of the skin (Figure 3) and spread over a 1" by 2" area using the smooth edge of the multiple puncture device (Figure 4).
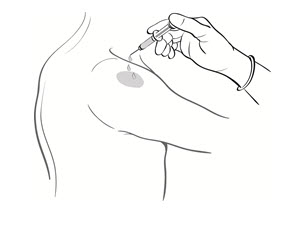
Figure 3
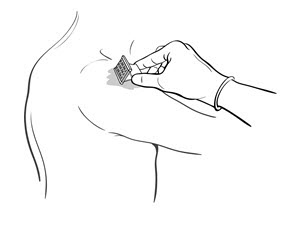
Figure 4
- 3.
- Grasp the arm firmly from underneath, tensing the skin. Center the multiple puncture device over the vaccine and apply firm downward pressure such that the device points are well buried in the skin (Figure 5).
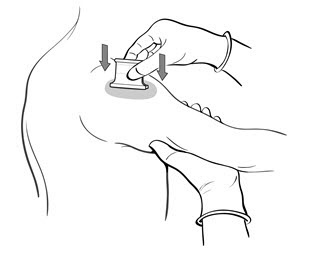
Figure 5
- 4.
- Maintain pressure for 5 seconds. Do not "rock" the device. Release the pressure underneath the arm and remove the device. In a successful procedure the points puncture the skin. If the points do not puncture the skin, the puncture procedure must be repeated.
- 5.
- After successful puncture, spread vaccine as evenly as possible over the puncture area with the smooth edge of the device (Figure 4). An additional 1–2 drops of BCG VACCINE may be added to ensure a very wet vaccination site.
- 6.
- Use the multiple puncture device once and discard in a standard biohazardous sharps container.
- 7.
- Loosely cover the site and keep dry for 24 hours.
- 8.
- Advise the patient that the vaccine contains live organisms. Although the vaccine will not survive in a dry state for long, infection of others is possible.
Tuberculin reactivity resulting from BCG vaccination should be documented. A vaccinated person should be tuberculin skin tested 2–3 months after BCG administration, and the test results, in millimeters of induration, should be recorded in the person's medical record.{9} Vaccination should be repeated for those who remain tuberculin negative to 5 TU of tuberculin after 2–3 months.
Pediatric Dose
Do not administer INTRAVENOUSLY, SUBCUTANEOUSLY, INTRAMUSCULARLY, OR INTRADERMALLY. Administer the vaccine in the deltoid region.
In infants less than 1 month old, the dosage of BCG VACCINE should be reduced by one-half, by using 2 mL of Sterile Water for Injection, USP at 4-25°C (39-77°F) when reconstituting. If a vaccinated infant remains tuberculin negative to 5 TU on skin testing, and if indications for vaccination persist, the infant should receive a full dose after 1 year of age.
-
HOW SUPPLIED
BCG VACCINE is supplied in a box of one single-dose vial of BCG. Each vial contains 1 to 8 × 108 CFU, which is equivalent to approximately 50 mg (wet weight), as lyophilized (freeze-dried) powder, NDC 0052-0603-02.
Multiple puncture devices may be obtained separately from the Order Management Center, Merck Sharp & Dohme LLC, 351 North Sumneytown Pike, North Wales, PA 19454-2505, Telephone number: 800-637-2579.
-
REFERENCES
- Guerin C: The history of BCG. In: Rosenthal SR (ed): BCG VACCINE: Tuberculosis-Cancer. Littleton, MA, PSG Publishing Co., Inc. 1980; pp. 35-43.
- Enarson, D, Murray, JF. Global Epidemiology of Tuberculosis. In: Rom, WN, Garay, SM (eds.); Tuberculosis. Boston, Little Brown and Company; 1996; pp. 57-76.
- Centers for Disease Control and Prevention. The Role of BCG VACCINE in the Prevention and Control of Tuberculosis in the United States. MMWR 45(RR-4):1-18, 1996.
- Centers for Disease Control and Prevention. Tuberculosis Morbidity – United States, 1998. MMWR 47(13):253-257.
- Moore M, Ornarato IM, McCray E, Castro KG. Trends in Drug-Resistant Tuberculosis in the United States, 1993-1996. JAMA 1997;278:833-837.
- Gordin FM, Matts JP, Miller C, Brown LS, Hafner R, John SL, et al: A Controlled Trial of Isoniazid in Persons with Anergy and Human Immunodeficiency Virus Infection Who are at High Risk for Tuberculosis. NEJM 1997;337:315-320.
- Comstock, GW. Field trials of tuberculosis vaccines: How could we have done better? Controlled Clinical Trials 1994;15:247-276.
- Colditz GA, Brewer TF, Berkey CS et al. Efficacy of BCG VACCINE in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 1994;271:698-709.
- Centers for Disease Control and Prevention. Use of BCG VACCINE in the Control of Tuberculosis. MMWR 43:663-675, 1988.
- Romanus V: Tuberculosis in bacillus Calmette-Guerin immunized and unimmunized children in Sweden: a ten-year evaluation following the cessation of general bacillus Calmette-Guerin immunization of the newborn in 1975. Pediatr Infect Dis 1987;6:272-280.
- Smith PG: Case-control studies of the efficacy of BCG against tuberculosis. In: International union against tuberculosis, Proceeding of the XXVIth IUAT World Conference on Tuberculosis and Respiratory Diseases, Singapore. Professional Postgraduate Services, International, Japan, 1987:73-79.
- Padungchan S, Konjanart S, Kasiratta S, et al: The effectiveness of BCG vaccination of the newborn against childhood tuberculosis in Bangkok. Bull WHO 1986;64:247-258.
- Tidjani O, Amedone A, ten Dam HG: The protective effect of BCG vaccination of the newborn against childhood tuberculosis in an African community. Tubercle 1986;67:269-281.
- Young TK, Hershfield ES: A case-control study to evaluate the effectiveness of mass neonatal BCG vaccination among Canadian Indians. Am J Public Health 1986;76:783-786.
- Shapiro C, Cook N, Evans D, et al.: A case-control study of BCG and childhood tuberculosis in Cali, Columbia. Int J Epidemiol 1985;14:441-446.
- Rosenthal SR, Loewinsohn E, Graham ML, Liveright D, Throne MG, Johnson V, Baston HC. BCG Vaccination Against Tuberculous in Chicago: a Twenty-year Study Statistically Analyzed. Pediatrics 1961;28:622-641.
- Data on file, Merck Teknika LLC, Durham, NC, USA.
- Lorin MI, Hsu KHK, Jacob SC: Treatment of tuberculosis in children. In: Symposium on anti-infective therapy. Pediatric Clinics of North America 1983;30:333-348.
- Stone MM, Vannier AM, Storch SK, et al.: Brief report: meningitis due to iatrogenic BCG infection in two immunocompromised children. Lancet 1995;333:561-563.
- Package Insert QuantiFERON®-TB Gold, Cellestis, Inc. July 2010.
- Package Insert T-SPOT®-TB. Oxford Immunotec Ltd, July 2010.
- Centers for Disease Control and Prevention. Guidelines for Preventing the Transmission of Mycobacterium Tuberculosis in Health-Care Facilities. MMWR 43:1-38, 1994.
- Report of the Committee on the Control of Infectious Diseases. American Academy of Pediatrics 1988; 21st Edition.
- Lotte A, Wazs-Hockert O, Poisson N, Dumitrescu N, Verron M, Couvet E: BCG Complications. Adv Tuberc Res 1984;21:107-193.
- Mande R: BCG Vaccination. Dawson of Pall Mall, London, UK, 1968, pp. 112-265.
- Griffith AH: Ten cases of BCG overdose treated with isoniazid. Tubercle 1963;44:247-250.
- Watkins SM: Unusual complications of BCG vaccination. Brit Med J 1971;1:442.
-
SPL UNCLASSIFIED SECTION
Manufactured for: Merck Sharp & Dohme LLC
Rahway, NJ 07065, USAManufactured by: Merck Teknika LLC, Durham, NC 27712, USA
U.S. License No. 1747
For patent information: www.msd.com/research/patent
TICE is a registered trademark of The Board of Trustees of the University of Illinois, used under the license of Merck Teknika LLC, Durham, NC, USA.
Copyright © 2021-2022 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.Revised: 08/2022
uspi-v914-pwi-2208r011
-
BCG VACCINE(for Percutaneous Use)
Instructions for Use
Please read this leaflet carefully before administering BCG VACCINE. The vaccine is to be administered after fully explaining the risks and benefits to the vaccinee, parent, or guardian. BCG vaccination should not be given to individuals previously infected with M. tuberculosis. The sterile, single-use multiple puncture device is for use with the BCG VACCINE only.
- 1.
- The vaccine should only be administered percutaneously utilizing the supplied sterile, single-use multiple puncture device. Do not inject intravenously, subcutaneously, intramuscularly, or intradermally. The multiple puncture device consists of a plastic holder for a thin, wafer-like stainless steel plate from which 36 points protrude (Figure 1).

Figure 1
- 2.
- After the vaccine is prepared, clean the skin site with an alcohol or acetone sponge and allow to dry thoroughly.
- 3.
- Administer the vaccine in the deltoid region (Figure 2). Position the arm to maintain a horizontal surface where the vaccine is to be placed.

Figure 2
- 4.
- Drop the immunizing dose of 0.2–0.3 mL of BCG VACCINE from the syringe and needle onto the cleansed surface of the skin (Figure 3) and spread over a 1" by 2" area using the smooth edge of the multiple puncture device (Figure 4).


Figure 3 Figure 4 - 5.
- Grasp the arm firmly from underneath, tensing the skin. Center the multiple puncture device over the vaccine and apply firm downward pressure such that the device points are well buried in the skin (Figure 5).

Figure 5
- 6.
- Maintain pressure for 5 seconds. Do not "rock" the device. Release the pressure underneath the arm and remove the device. In a successful procedure the points puncture the skin. If the points do not puncture the skin, the puncture procedure must be repeated.
- 7.
- After successful puncture, spread vaccine as evenly as possible over the puncture area with the smooth edge of the device (Figure 4). An additional 1–2 drops of BCG VACCINE may be added to ensure a very wet vaccination site.
- 8.
- Use the multiple puncture device once and discard in a standard biohazardous sharps container.
- 9.
- Loosely cover the site and keep dry for 24 hours.
- 10.
- Advise the patient that the vaccine contains live organisms. Although the vaccine will not survive in a dry state for long, infection of others is possible.
Caution: Federal law restricts this device to sale by or on the order of a physician.
Multiple puncture devices may be obtained separately from the Order Management Center, Merck Sharp & Dohme LLC, 351 North Sumneytown Pike, North Wales, PA 19454-2505, Telephone number: 800-637-2579.
-
SPL UNCLASSIFIED SECTION
Manufactured for:
Merck Teknika LLC, Durham, NC 27712, USAFor patent information: www.msd.com/research/patent
Copyright © 2021-2022 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.Revised:08/2022
ifu-v914-pwi-2208r008
- PRINCIPAL DISPLAY PANEL - Vial Carton
-
INGREDIENTS AND APPEARANCE
BCG VACCINE
bacillus calmette-guerin substrain tice live antigen injection, powder, lyophilized, for solutionProduct Information Product Type VACCINE Item Code (Source) NDC:0052-0603 Route of Administration PERCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACILLUS CALMETTE-GUERIN SUBSTRAIN TICE LIVE ANTIGEN (UNII: 2XQ558L16Z) (BACILLUS CALMETTE-GUERIN SUBSTRAIN TICE LIVE ANTIGEN - UNII:2XQ558L16Z) BACILLUS CALMETTE-GUERIN SUBSTRAIN TICE LIVE ANTIGEN 50 mg Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Water (UNII: 059QF0KO0R) glycerin (UNII: PDC6A3C0OX) asparagine (UNII: 5Z33R5TKO7) citric acid monohydrate (UNII: 2968PHW8QP) potassium phosphate, unspecified form (UNII: B7862WZ632) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) ferric ammonium citrate (UNII: UVP74NG1C5) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0052-0603-02 1 in 1 CARTON 1 NDC:0052-0603-01 1 in 1 VIAL; Type 5: Device Coated or Otherwise Combined with Biologic Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103050 06/21/1989 Labeler - Merck Sharp & Dohme LLC (118446553)