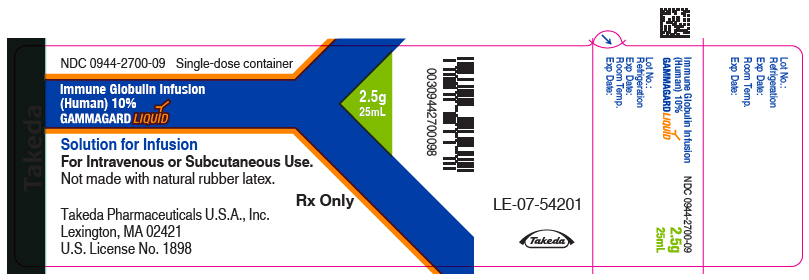
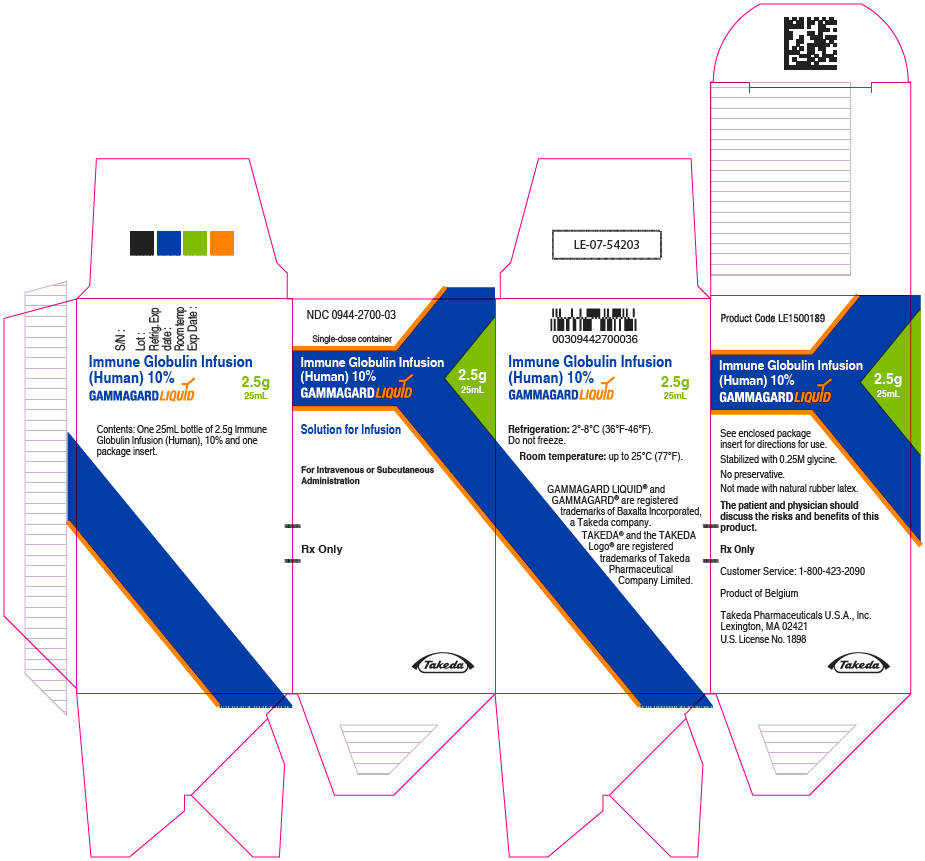
Label: GAMMAGARD LIQUID (immune globulin infusion- human injection, solution
-
NDC Code(s):
0944-2700-02,
0944-2700-03,
0944-2700-04,
0944-2700-05, view more0944-2700-06, 0944-2700-07, 0944-2700-08, 0944-2700-09, 0944-2700-10, 0944-2700-11, 0944-2700-12, 0944-2700-13
- Packager: Takeda Pharmaceuticals America, Inc.
- Category: PLASMA DERIVATIVE
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated January 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GAMMAGARD LIQUID safely and effectively. See full prescribing information for GAMMAGARD LIQUID.
GAMMAGARD LIQUID, Immune Globulin Infusion (Human), 10% Solution, for intravenous and subcutaneous administration
Initial U.S. Approval: 2005WARNING: THROMBOSIS, RENAL DYSFUNCTION and ACUTE RENAL FAILURE
See full prescribing information for complete boxed warning.
- ▪
- Thrombosis may occur with immune globulin products, including GAMMAGARD LIQUID. Risk factors may include advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling vascular catheters, hyperviscosity and cardiovascular risk factors. (5.4)
- ▪
- Renal dysfunction, acute renal failure, osmotic nephrosis, and death may occur in predisposed patients with immune globulin intravenous (IGIV) products including GAMMAGARD LIQUID. Renal dysfunction and acute failure occur more commonly with IGIV products containing sucrose. GAMMAGARD LIQUID does not contain sucrose. (5.2)
- ▪
- For patients at risk of thrombosis, administer GAMMAGARD LIQUID at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk of hyperviscosity. (5.4)
INDICATIONS AND USAGE
GAMMAGARD LIQUID is an immune globulin infusion (human) indicated as a:
- ▪
- Replacement therapy for primary humoral immunodeficiency (PI) in adult and pediatric patients two years of age or older. (1.1)
- ▪
- Maintenance therapy to improve muscle strength and disability in adult patients with Multifocal Motor Neuropathy (MMN). (1.2)
- ▪
- Therapy to improve neuromuscular disability and impairment in adult patients with Chronic Inflammatory Demyelinating Polyneuropathy (CIDP). (1.3)
Limitations of Use:
DOSAGE AND ADMINISTRATION
PI: Intravenous (IV) (2.1)
Dose Initial Infusion Rate Maintenance Infusion Rate 300 to 600 milligram (mg)/kg every 3 to 4 weeks based on clinical response 0.5milliliter (mL)/kg/hr
(0.8 mg/kg/min) for 30 minutesIncrease every 30minutes (if tolerated) up to 5 mL/kg/hr (8 mg/kg/min) Subcutaneous (SC) (2.1)
Dose Initial Infusion Rate Maintenance Infusion Rate Initial Dose is 1.37 × previous intravenous dose divided by # of weeks between intravenous doses.
Maintenance dose is based on clinical response and target IgG trough level.40 kg BW and greater: 30 mL/site at 20 mL/hr/site
Under 40 kg BW: 20 mL/site at 15 mL/hr/site40 kg BW and greater: 30 mL/site at 20 to 30 mL/hr/site
Under 40 kg BW: 20 mL/site at 15 to 20 mL/hr/siteMMN: Intravenous (IV) (2.2)
Dose Initial Infusion Rate Maintenance Infusion Rate Dose range 0.5 to 2.4 gram/kg/month based on clinical response 0.5mL/kg/hr
(0.8 mg/kg/min)Infusion rate may be advanced if tolerated to 5.4 mL/kg/hr (9 mg/kg/min) CIDP: Intravenous (IV) (2.3)
Dose Initial Infusion Rate Maintenance Infusion Rate Induction dose is 2 g/kg in divided doses over 2 to 5 consecutive days, followed by maintenance infusions.
Maintenance dose is 1 g/kg in divided doses over 1 to 4 consecutive days, every 3 weeks.0.5 mL/kg/hr
(0.8 mg/kg/min)Infusion rate may be increased if tolerated up to 5.4 mL/kg/hr (9 mg/kg/min) - ▪
- Ensure patients with pre-existing renal insufficiency are not volume depleted; discontinue GAMMAGARD LIQUID if renal function deteriorates. (2.5, 5.2)
- ▪
- For patients at risk of renal dysfunction or thrombotic events, administer GAMMAGARD LIQUID at minimum infusion rate practicable. (2.5, 5.2, 5.4)
DOSAGE FORMS AND STRENGTHS
Aqueous solution containing 10% IgG (100 mg/mL) (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- ▪
- IgA deficient patients with antibodies to IgA are at greater risk of developing severe hypersensitivity and anaphylactic reaction. (5.1)
- ▪
- Monitor renal function, including blood urea nitrogen, serum creatinine, and urine output in patients at risk of acute renal failure. (5.2)
- ▪
- Hyperproteinemia, increased serum viscosity and hyponatremia may occur. (5.3)
- ▪
- Thrombosis may occur. Monitor for signs and symptoms of thrombosis and assess blood viscosity for those at risk for hyperviscosity. (5.4)
- ▪
- Aseptic Meningitis Syndrome (AMS) may occur. (5.5)
- ▪
- Hemolytic anemia can develop. Monitor for clinical signs and symptoms of hemolysis and hemolytic anemia. (5.6)
- ▪
- Monitor patients for pulmonary adverse reactions (transfusion-related acute lung injury, TRALI). (5.7)
- ▪
- GAMMAGARD LIQUID is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and theoretically, the Creutzfeldt-Jakob disease agent (5.8)
ADVERSE REACTIONS
The most common adverse reactions observed in ≥5% of subjects were:
PI: Intravenous Administration: Headache, fatigue, pyrexia, nausea, chills, rigors, pain in extremity, diarrhea, migraine, dizziness, vomiting, cough, urticaria, asthma, pharyngolaryngeal pain, rash, arthralgia, myalgia, oedema peripheral, pruritus, and cardiac murmur. (6.1)
Subcutaneous Administration: Infusion site (local) event, headache, fatigue, heart rate increased, pyrexia, abdominal pain upper, nausea, vomiting, asthma, blood pressure systolic increased, diarrhea, ear pain, aphthous stomatitis, migraine, oropharyngeal pain, and pain in extremity. (6.1)
MMN: Headache, chest discomfort, muscle spasms, muscular weakness, nausea, oropharyngeal pain, and pain in extremity. (6.1)
CIDP: Headache, pyrexia anemia, leukopenia, neutropenia, illness, blood creatinine increased, dizziness, migraine, somnolence, tremor, nasal dryness, abdominal pain upper, vomiting, chills, nasopharyngitis, and pain in extremity. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals U.S.A., Inc. at 1-877-TAKEDA-7 (1-877-825-3327) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Passive transfer of antibodies may transiently interfere with immune responses to live virus vaccines, such as measles, mumps, rubella, and varicella. (7)
USE IN SPECIFIC POPULATIONS
Geriatric: In patients over age 65 or in any patient at risk of developing renal insufficiency, do not exceed the recommended dose. Infuse GAMMAGARD LIQUID at the minimum infusion rate practicable. (8.5)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: THROMBOSIS, RENAL DYSFUNCTION and ACUTE RENAL FAILURE
1 INDICATIONS AND USAGE
1.1 Primary Immunodeficiency (PI)
1.2 Multifocal Motor Neuropathy (MMN)
1.3 Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
2 DOSAGE AND ADMINISTRATION
2.1 Dosage for Primary Immunodeficiency
2.2 Dosage for Multifocal Motor Neuropathy
2.3 Dosage for Chronic Inflammatory Demyelinating Polyneuropathy
2.4 Preparation and Handling
2.5 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity Reaction to Immune Globulins
4.2 IgA Sensitive Patients with History of Hypersensitivity Reactions
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
5.2 Renal Dysfunction/Failure
5.3 Hyperproteinemia, Increased Serum Viscosity, and Hyponatremia
5.4 Thrombosis
5.5 Aseptic Meningitis Syndrome (AMS)
5.6 Hemolysis
5.7 Transfusion-Related Acute Lung Injury (TRALI)
5.8 Transmissible Infectious Agents
5.9 Monitoring: Laboratory Tests
5.10 Interference with Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: THROMBOSIS, RENAL DYSFUNCTION and ACUTE RENAL FAILURE
- ▪
- Thrombosis may occur with immune globulin products, including GAMMAGARD LIQUID. Risk factors may include advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling vascular catheters, hyperviscosity and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors [see Warnings and Precautions (5.4), Patient Counseling Information (17)].
- ▪
- Renal dysfunction, acute renal failure, osmotic nephrosis, and death may occur in predisposed patients with immune globulin intravenous (IGIV) products. Patients predisposed to renal dysfunction include those with any degree of pre-existing renal insufficiency, diabetes mellitus, age greater than 65, volume depletion, sepsis, paraproteinemia, or patients receiving known nephrotoxic drugs. Renal dysfunction and acute renal failure occur more commonly in patients receiving IGIV products containing sucrose. GAMMAGARD LIQUID does not contain sucrose. [see Warnings and Precautions (5.2)
- ▪
- For patients at risk of thrombosis, administer GAMMAGARD LIQUID at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk of hyperviscosity [see Dosage and Administration (2.3) and Warnings and Precautions (5.4)].
-
1 INDICATIONS AND USAGE
1.1 Primary Immunodeficiency (PI)
GAMMAGARD LIQUID is indicated as replacement therapy for primary humoral immunodeficiency (PI) in adult and pediatric patients two years of age or older. This includes, but is not limited to, common variable immunodeficiency (CVID), X-linked agammaglobulinemia, congenital agammaglobulinemia, Wiskott-Aldrich syndrome, and severe combined immunodeficiencies.1,2
1.2 Multifocal Motor Neuropathy (MMN)
GAMMAGARD LIQUID is indicated as a maintenance therapy to improve muscle strength and disability in adult patients with Multifocal Motor Neuropathy (MMN).
1.3 Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
GAMMAGARD LIQUID is indicated as a therapy to improve neuromuscular disability and impairment in adult patients with Chronic Inflammatory Demyelinating Polyneuropathy (CIDP).
Limitation of Use
GAMMAGARD LIQUID has not been studied in immunoglobulin-naive patients with CIDP. GAMMAGARD LIQUID maintenance therapy in CIDP has not been studied for periods longer than 6 months. After responding during an initial treatment period, not all patients require indefinite maintenance therapy with GAMMAGARD LIQUID in order to remain free of CIDP symptoms. Individualize the duration of any treatment beyond 6 months based upon the patient’s response and demonstrated need for continued therapy.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage for Primary Immunodeficiency
Intravenous Administration (IV)
Table 1: Recommended Dosage for Primary Immunodeficiency (Intravenous Administration) Dose Initial Infusion rate Maintenance Infusion rate 300 to 600 milligram (mg)/kg every 3 to 4 weeks based on clinical response 0.5 milliliter (mL)/kg/hr
(0.8mg/kg/min) for 30 minutesIncrease every 30 minutes (if tolerated) up to 5 mL/kg/hr (8mg/kg/min) Dose Adjustments
Adjust dose according to IgG levels and clinical response, as the frequency and dose of immune globulin may vary from patient to patient.
No randomized controlled clinical studies are available to determine an optimum trough serum IgG level for intravenous treatment. If a patient misses a dose, administer the missed dose as soon as possible, and then resume scheduled treatments every 3 or 4 weeks, as applicable.
Prior to switching from intravenous to subcutaneous treatment, obtain the patient's serum IgG trough level to guide subsequent dose adjustments. Start the initial subcutaneous dose approximately one week after the last intravenous infusion.
Dose Adjustments for Measles Exposure
If a patient has been exposed to measles, it may be prudent to administer an extra dose of GAMMAGARD LIQUID as soon as possible and within 6 days of exposure. A dose of 400 mg/kg should provide a serum level > 240 mIU/mL of measles antibodies for at least two weeks.
If a patient is at risk of future measles exposure and receives a dose of less than 530 mg/kg every 3 to 4 weeks, the dose should be increased to at least 530 mg/kg. This should provide a serum level of 240 mIU/mL of measles antibodies for at least 22 days after infusion.
Subcutaneous Administration (SC)
Table 2: Recommended Dosage for Primary Immunodeficiency (Subcutaneous Administration) Dose Initial Infusion rate Maintenance Infusion rate Initial dose is 1.37 × previous intravenous dose divided by # of weeks between intravenous doses.
Maintenance dose is based on clinical response and target IgG trough level.40 kg BW and greater: 30 mL/site at 20 mL/hr/site.
Under 40 kg BW: 20 mL/site at 15 mL/hr/site.40 kg BW and greater: 30 mL/site at 20 to 30 mL/hr/site.
Under 40 kg BW: 20 mL/site at 15 to 20 mL/hr/site.Dose Adjustments
Based on the results of clinical studies, the expected increase in serum IgG trough level during weekly subcutaneous treatment at the dose adjusted to provide a comparable AUC, is approximately 281 mg/dL higher than the last IgG trough level during prior stable intravenous treatment. To calculate the target trough IgG level for subcutaneous treatment, add 281 mg/dL to the IgG trough level obtained after the last intravenous treatment.
To guide dose adjustment, calculate the difference between the patient's target serum IgG trough level and the IgG trough level during subcutaneous treatment. Find this difference in the columns of Table 3 and the corresponding amount (in mL) by which to increase (or decrease) the weekly dose based on the patient's body weight. If the difference between measured and target trough levels is less than 100 mg/dL then no adjustment is necessary. However, the patient's clinical response should be the primary consideration in dose adjustment.
Table 3: Change in Weekly Dose of GAMMAGARD LIQUID for Intended IgG Trough Level Adjustment* - *
- Derived using a linear approximation to the nomogram method with a slope of 5.3 kg/dL.
Difference between Measured and Target IgG Trough Levels
Body Weight
100 mg/dL
200 mg/dL
300 mg/dL
400 mg/dL
10 kg
2 mL
4 mL
6 mL
8 mL
20 kg
4 mL
8 mL
11 mL
15 mL
30 kg
6 mL
11 mL
17 mL
23 mL
40 kg
8 mL
15 mL
23 mL
30 mL
50 kg
9 mL
19 mL
28 mL
38 mL
60 kg
11 mL
23 mL
34 mL
45 mL
70 kg
13 mL
26 mL
40 mL
53 mL
80 kg
15 mL
30 mL
45 mL
60 mL
90 kg
17 mL
34 mL
51 mL
68 mL
100 kg
19 mL
38 mL
57 mL
75 mL
110 kg
21 mL
42 mL
62 mL
83 mL
120 kg
23 mL
45 mL
68 mL
91 mL
130 kg
25 mL
49 mL
74 mL
98 mL
140 kg
26 mL
53 mL
79 mL
106 mL
Example 1: A patient with a body weight of 80 kg has a measured IgG trough level of 800 mg/dL and the target trough level is 1000 mg/dL. The desired target trough level difference is 200 mg/dL (1000 mg/dL minus 800 mg/dL). The weekly dose of GAMMAGARD LIQUID should be increased by 30 mL (3.0 gm).
Example 2: A patient with a body weight of 60 kg has a measured IgG trough of 1000 mg/dL and the target trough level is 800 mg/dL. The desired target trough level difference is 200 mg/dL (800 mg/dL minus 1000 mg/dL). The weekly dose of GAMMAGARD LIQUID should be decreased by 23 mL (2.3 gm).
2.2 Dosage for Multifocal Motor Neuropathy
Intravenous Administration (IV)
Table 4: Recommended Dosage for Multifocal Motor Neuropathy Dose Initial Infusion rate Maintenance Infusion rate Dose range 0.5 to 2.4 gram/kg/month based on clinical response (14) 0.5 mL/kg/hr
(0.8 mg/kg/min)Infusion rate may be increased if tolerated up to 5.4 mL/kg/hr (9 mg/kg/min) Dose Adjustments
The dose may need to be adjusted to achieve the desired clinical response. In the clinical study, the dose ranged between 0.5 to 2.4 grams/kg/month. While receiving GAMMAGARD LIQUID, 9% of subjects in the clinical study experienced neurological decompensation that required an increase in dose. In order to avoid worsening of muscle weakness in patients, dose adjustment may be necessary.
2.3 Dosage for Chronic Inflammatory Demyelinating Polyneuropathy
Intravenous Administration (IV)
Table 5: Recommended Dosage for Chronic Inflammatory Demyelinating Polyneuropathy Dose Initial Infusion rate Maintenance Infusion rate Induction dose is 2 g/kg in divided doses over 2 to 5 consecutive days, followed by maintenance infusions.
Maintenance dose is 1 g/kg in divided doses over 1 to 4 consecutive days, every 3 weeks.0.5 mL/kg/hr
(0.8 mg/kg/min)Infusion rate may be increased if tolerated up to 5.4 mL/kg/hr (9 mg/kg/min) Dose Adjustments
The induction dose is 2 g/kg divided over 2 to 5 consecutive days, followed by 1 g/kg maintenance doses divided over 1 to 4 days consecutive days, every 3 weeks. The maintenance dose level and dosing interval of GAMMAGARD LIQUID treatment may be adjusted according to the clinical response.
The recommended initial infusion rate is 0.5 mL/kg/hr (0.8 mg/kg/min). If the infusion is well tolerated, the rate may be gradually increased to a maximum of 5.4 mL/kg/hr (9 mg/kg/min). [see Administration (2.5)].
For patients at risk of renal dysfunction or thromboembolic events, do not exceed the recommended dose. Infuse at the minimum intravenous infusion rate practicable [see Warnings and Precautions (5.2, 5.4) and Use In Specific Populations (8.5)].
2.4 Preparation and Handling
- ▪
- Inspect the drug product visually for particulate matter and discoloration prior to administration. GAMMAGARD LIQUID is a clear or slightly opalescent, colorless or pale-yellow solution. Do not use if the solution is cloudy, turbid, or if it contains particulates.
- ▪
- GAMMAGARD LIQUID vial is for single use only. Any vial that has been entered should be used promptly. Partially used vials should be discarded. GAMMAGARD LIQUID contains no preservative.
- ▪
- Allow refrigerated product to come to room temperature before use. DO NOT MICROWAVE.
- ▪
- Do not shake.
- ▪
- Do not mix with other products.
- ▪
- Do not use normal saline as a diluent. If dilution is desired, 5% dextrose in water (D5W) should be used as a diluent.
- ▪
- The infusion line may be flushed with normal saline. An in-line filter is optional.
- ▪
- Record the name and lot number of the product in the recipient's records.
2.5 Administration
Intravenous administration
Table 6: Infusion Rates for Intravenous Administration PI
MMN
CIDP
Initial
0.5 mL/kg/hr
(0.8 mg/kg/min) for 30 minutesIncreasing rates of infusion starting at 0.5 mL/kg/h (0.8 mg/kg/min)
0.5 mL/kg/hr (0.8 mg/kg/min)
Subsequent
Increase every 30 minutes (if tolerated) up to 5 mL/kg/hr (8 mg/kg/min)
Increasing to a maximum rate of 5.4 mL/kg/hr if tolerated (9 mg/kg/min)
Increase to a maximum rate of 5.4 mL/kg/hr if tolerated (9 mg/kg/min)
Monitor patient vital signs throughout the infusion. Certain adverse reactions (ARs) such as headaches, flushing, and changes in pulse rate and blood pressure may be related to the rate of infusion. Slow or stop infusion if adverse reactions occur. If symptoms subside promptly, the infusion may be resumed at a lower rate that does not result in recurrence of the symptoms.
Adverse reactions may occur more frequently in patients receiving immune globulin for the first time, upon switching brands or if there has been a long interval since the previous infusion.2 In such cases, start at lower infusion rates and gradually increase as tolerated.
Ensure that patients with pre-existing renal insufficiency are not volume depleted. For patients over 65 years of age or judged to be at risk for renal dysfunction or thrombotic events, administer GAMMAGARD LIQUID at the minimum infusion rate practicable. In such cases, the maximal rate should be less than 3.3 mg/kg/min (<2 mL/kg/hr); consider discontinuation of administration if renal function deteriorates [see Warnings and Precautions (5.2, 5.4) and Use In Specific Populations (8.5)].
Subcutaneous administration for PI
Table 7: Infusion Rates for Subcutaneous Administration 40 kg BW and greater
Under 40 kg BW
Initial
30 mL/site at a rate of 20 mL/hr/site
20 mL/site at a rate of 15 mL/hr/site
Maintenance
30 mL/site at a rate of 20 to 30 mL/hr/site
20 mL/site at a rate of 15 to 20 mL/hr/site
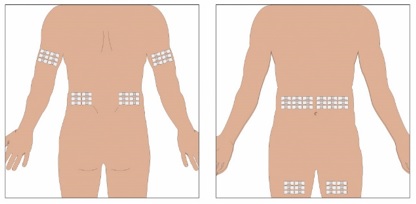
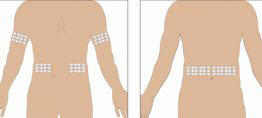
Selection of Infusion Site: Suggested areas for subcutaneous infusion of GAMMAGARD LIQUID are abdomen, thighs, upper arms, or lower back. Infusion sites should be at least two inches apart, avoiding bony prominences. Rotate sites each week.
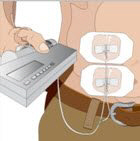
Volume per Site: The weekly dose (mL) should be divided by 30 or 20, based on patient weight above, to determine the number of sites required. Simultaneous subcutaneous infusion at multiple sites can be facilitated by use of a multi-needle administration set.
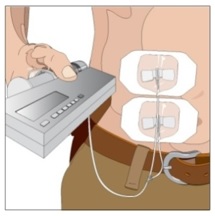
Rate of Infusion for Patients 40 kg and greater (88 lbs): If multiple sites are used, the rate set on the pump should be the rate per site multiplied by the number of sites (e.g., 30 mL x 4 sites = 120 mL/hr). The number of simultaneous sites should be limited to 8, or maximum infusion rate of 240 mL/hr.
Rate of Infusion for Patients under 40 kg (88 lbs): If multiple sites are used, the rate set on the pump should be the rate per site multiplied by the number of sites (e.g., 20 mL x 3 sites = 60 mL/hr). The number of simultaneous sites should be limited to 8, or maximum infusion rate of 160 mL/hr.
Instructions for Subcutaneous Administration: Instruct patients to observe the following procedures:
- 1.
- Aseptic technique - Use aseptic technique when preparing and infusing GAMMAGARD LIQUID.
- 2.
- Assemble supplies - Set up a clean work area and gather all supplies necessary for the subcutaneous infusion: vial(s) of GAMMAGARD LIQUID, ancillary supplies, sharps container and pump. If GAMMAGARD LIQUID has already been pooled into a bag or a syringe, skip to Step 5.
- 3.
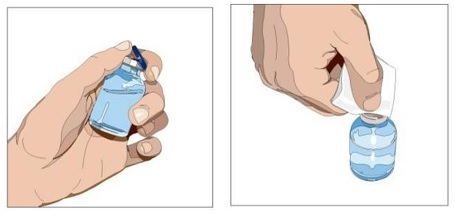
- Product preparation - Remove the protective cap from the vial to expose the center of the vial. Wipe the stopper with an alcohol pad and allow to dry.
- 4.
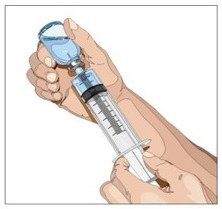
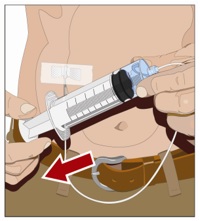
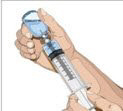
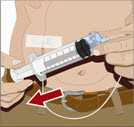
- Withdraw GAMMAGARD LIQUID from the vials - Attach a sterile syringe to a needle and draw air into the syringe barrel equal to the amount of product to be withdrawn. Inject the air into the vial and withdraw the desired volume of GAMMAGARD LIQUID. If multiple vials are required to achieve the desired dose, repeat this step.
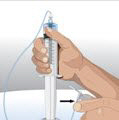
- 5.
- Prepare the infusion pump and tubing - Follow the manufacturer's instructions for preparing the pump and administration tubing, if needed. Be sure to prime the pump tubing to ensure that no air is left in the tubing and needle.
- 6.
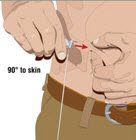
- Select the infusion sites - Select the number of infusion sites depending on the volume of the total dose. [See Dosage and Administration (2.5)] for recommended maximum volumes and rates. Potential sites for infusion include the back of arms, abdomen, thighs, and lower back (see Figure below). Ensure sites are at least 2 inches apart; avoid bony prominences.
- 7.
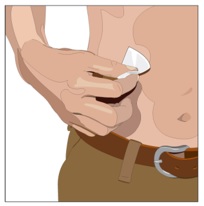
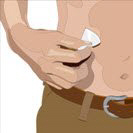
- Cleanse the infusion site(s) - Cleanse the infusion site(s) with an antiseptic skin preparation (e.g., alcohol pad) using a circular motion working from the center of the site and moving to the outside. Allow to dry.
- 8.
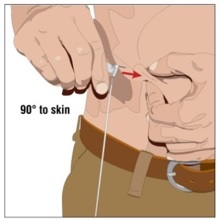
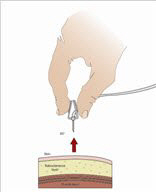
- Insert the needle - Choose the correct needle length to assure that GAMMAGARD LIQUID is delivered into the subcutaneous space. Grasp the skin and pinch at least one inch of skin between two fingers. Insert needle at a 90-degree angle with a darting motion into the subcutaneous tissue. Secure the needle.
- 9.
- Check for proper needle placement - Prior to the start of infusion, check each needle for correct placement to make sure that a blood vessel has not been punctured. Gently pull back on the attached syringe plunger and monitor for any blood return in the needle set. If you see any blood, remove and discard the needle set. Repeat priming and needle insertion steps in a different infusion site with a new needle set.
- 10.
- Secure the needle to the skin - Secure the needle(s) in place by applying a sterile protective dressing over the site.
- 11.
- Start infusion of GAMMAGARD LIQUID - Follow the manufacturer's instructions to turn pump on.
- 12.
- Document the infusion - Remove the peel-off label with product lot number and expiration date from the GAMMAGARD LIQUID vial and place in treatment diary/logbook to keep track of the product lots used. Keep the treatment diary/logbook current by recording the time, date, dose, product label and any reactions after each infusion.
- 13.
- Remove needle set - After the infusion is complete, remove the needle set and gently press a small piece of gauze over the needle insertion site and cover with a protective dressing. Discard any unused solution and disposable supplies in accordance with local requirements.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Hypersensitivity Reaction to Immune Globulins
GAMMAGARD LIQUID is contraindicated in patients who have a history of anaphylactic or severe systemic hypersensitivity reactions to administration of human immune globulin.
4.2 IgA Sensitive Patients with History of Hypersensitivity Reactions
GAMMAGARD LIQUID is contraindicated in IgA-deficient patients with antibodies to IgA and a history of hypersensitivity. Anaphylaxis has been reported with intravenous use of GAMMAGARD LIQUID and is theoretically possible following subcutaneous administration [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
Severe hypersensitivity reactions may occur, even in patients who had tolerated previous treatment with human normal immune globulin. In case of hypersensitivity, discontinue GAMMAGARD LIQUID infusion immediately and institute appropriate treatment.
GAMMAGARD LIQUID contains trace amount of IgA (average concentration of 37 μg/mL). Patients with antibodies to IgA have a greater risk of developing potentially severe hypersensitivity and anaphylactic reactions. GAMMAGARD LIQUID is contraindicated in patients with antibodies against IgA and a history of hypersensitivity reaction [see Contraindications (4.1)].
5.2 Renal Dysfunction/Failure
Acute renal dysfunction/failure, acute tubular necrosis, proximal tubular nephropathy, osmotic nephrosis, and death may occur upon use of IGIV treatment, especially those containing sucrose3. Acute renal dysfunction/failure has been reported in association with infusions of GAMMAGARD LIQUID. Assure that patients are not volume depleted prior to the initiation of infusion of GAMMAGARD LIQUID. In patients who are at risk of developing renal dysfunction because of pre-existing renal insufficiency or predisposition to acute renal failure (such as diabetes mellitus, age greater than 65, volume depletion, sepsis, paraproteinemia, or patients receiving known nephrotoxic drugs, etc.), administer GAMMAGARD LIQUID intravenously at the minimum rate of infusion practicable (not exceeding 3.3 mg IgG/kg/min (<2 mL/kg/hr) [see Dosage and Administration (2.5)].
Periodic monitoring of renal function and urine output is particularly important in patients judged to be at increased risk for developing acute renal failure. Assess renal function, including measurement of blood urea nitrogen (BUN) and serum creatinine, before the initial infusion of GAMMAGARD LIQUID and again at appropriate intervals thereafter. If renal function deteriorates, consider discontinuation of GAMMAGARD LIQUID [see Dosage and Administration (2.5)].
5.3 Hyperproteinemia, Increased Serum Viscosity, and Hyponatremia
Hyperproteinemia, increased serum viscosity and hyponatremia may occur in patients receiving GAMMAGARD LIQUID. It is critical to distinguish true hyponatremia from pseudohyponatremia that is temporally or causally related to hyperproteinemia with concomitant decreased calculated serum osmolality or elevated osmolar gap. Treatment aimed at decreasing serum free water in patients with pseudohyponatremia may lead to volume depletion, a further increase in serum viscosity, and a predisposition to thromboembolic events.4
5.4 Thrombosis
Thrombosis may occur following treatment with immune globulin products, including GAMMAGARD LIQUID. Risk factors may include advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors.
Consider baseline assessment of blood viscosity in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies. For patients at risk of thrombosis, administer GAMMAGARD LIQUID at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity [see Boxed Warning, Dosage and Administration (2.5), Patient Counseling Information (17)].
5.5 Aseptic Meningitis Syndrome (AMS)
AMS may occur with immune globulin treatment, including GAMMAGARD LIQUID, administered intravenously or subcutaneously. AMS may occur more frequently in female patients. Discontinuation of immune globulin treatment has resulted in remission of AMS within several days without sequelae. The syndrome usually begins within several hours to two days following immune globulin treatment.
AMS is characterized by the following signs and symptoms: severe headache, nuchal rigidity, drowsiness, fever, photophobia, painful eye movements, nausea and vomiting [see Patient Counseling Information (17)]. Cerebrospinal fluid (CSF) studies frequently reveal pleocytosis up to several thousand cells per mm3, predominantly from the granulocytic series, and elevated protein levels up to several hundred milligram/dL, but negative culture results. Conduct a thorough neurological examination on patients exhibiting such symptoms and signs, including CSF studies, to rule out other causes of meningitis.
5.6 Hemolysis
GAMMAGARD LIQUID, contains blood group antibodies that may act as hemolysins and induce in vivo coating of red blood cells (RBC) with immune globulin. This may cause a positive direct antiglobulin test [DAT (Coomb's test)].5,6 Delayed hemolytic anemia can develop subsequent to GAMMAGARD LIQUID therapy due to enhanced RBC sequestration; acute hemolysis, consistent with intravascular hemolysis, has been reported [see Adverse Reactions (6)].5-8
The following risk factors may be related to the development of hemolysis: high doses (e.g., ≥2 grams/kg, single administration or divided over several days) and non-O blood group.5 Underlying inflammatory state in an individual patient may increase the risk of hemolysis,5 but its role is uncertain.8,9
Monitor patients for clinical signs and symptoms of hemolysis particularly patients with risk factors noted above. Consider appropriate laboratory testing in higher risk patients, including measurement of hemoglobin or hematocrit prior to infusion and within approximately 36 to 96 hours post infusion. If clinical signs and symptoms of hemolysis or a significant drop in hemoglobin or hematocrit have been observed, perform additional confirmatory laboratory testing. If transfusion is indicated for patients who develop hemolysis with clinically compromising anemia after receiving IGIV, perform adequate cross-matching to avoid exacerbating on-going hemolysis [see Warnings and Precautions (5.9)].
5.7 Transfusion-Related Acute Lung Injury (TRALI)
Non-cardiogenic pulmonary edema (TRALI) has been reported in patients following treatment with IGIV products, including GAMMAGARD LIQUID. TRALI is characterized by severe respiratory distress, pulmonary edema, hypoxemia, normal left ventricular function, and fever. Symptoms typically occur within 1 to 6 hours after treatment.
Monitor patients for pulmonary adverse reactions [see Patient Counseling Information (17)]. If TRALI is suspected, perform appropriate tests for the presence of anti-neutrophil and anti-HLA antibodies in both the product and patient serum. TRALI may be managed using oxygen therapy with adequate ventilatory support.
5.8 Transmissible Infectious Agents
Because GAMMAGARD LIQUID is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and theoretically, the Creutzfeldt-Jakob disease agent. This also applies to unknown or emerging viruses and other pathogens. No confirmed cases of viral transmission or vCJD have been associated with GAMMAGARD LIQUID.
All infections thought by a physician to possibly have been transmitted by this product should be reported by the physician or other healthcare provider to Takeda Pharmaceuticals U.S.A., Inc. at 1-877-TAKEDA-7 (1-877-825-3327) (in the U.S.).
5.9 Monitoring: Laboratory Tests
- ▪
- Periodic monitoring of renal function and urine output is particularly important in patients judged to be at increased risk of developing acute renal failure. Assess renal function, including measurement of blood urea nitrogen (BUN) and serum creatinine, before the initial infusion of GAMMAGARD LIQUID and at appropriate intervals thereafter.
- ▪
- Consider baseline assessment of blood viscosity in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies, because of the potentially increased risk of thrombosis.3,4
- ▪
- If signs and/or symptoms of hemolysis are present after an infusion of GAMMAGARD LIQUID, perform appropriate laboratory testing for confirmation.
- ▪
- If TRALI is suspected, perform appropriate tests for the presence of anti-neutrophil antibodies and anti-HLA antibodies in both the product and the patient's serum.
5.10 Interference with Laboratory Tests
After infusion of IgG, the transitory rise of the various passively transferred antibodies in the patient's blood may yield false positive serological testing results, with the potential for misleading interpretation. Passive transmission of antibodies to erythrocyte antigens (e.g., A, B, and D) may cause a positive direct or indirect antiglobulin (Coombs') test.
Administration of immune globulin products, including GAMMAGARD LIQUID, can lead to false positive readings in assays that depend on detection of beta-D-glucans for diagnosis of fungal infections; this may persist during the weeks following infusion of the product.
-
6 ADVERSE REACTIONS
Primary Immunodeficiency (PI)
Intravenous administration:
The serious adverse reaction (AR) seen during intravenous treatment in the clinical studies for PI was aseptic meningitis. The most common adverse reactions for PI (observed in ≥5% of subjects) were headache, fatigue, pyrexia, nausea, chills, rigors, pain in extremity, diarrhea, migraine, dizziness, vomiting, cough, urticaria, asthma, pharyngolaryngeal pain, rash, arthralgia, myalgia, oedema peripheral, pruritus, and cardiac murmur.
Subcutaneous Administration:
No serious adverse reactions were observed during the clinical study of subcutaneous treatment. The most common adverse reactions during subcutaneous treatment (observed in ≥5% of PI subjects) were infusion site (local) event, headache, fatigue, heart rate increased, pyrexia, abdominal pain upper, nausea, vomiting, asthma, blood pressure systolic increased, diarrhea, ear pain, aphthous stomatitis, migraine, oropharyngeal pain, and pain in extremity.
Multifocal Motor Neuropathy (MMN)
The serious adverse reactions in the clinical study for MMN were pulmonary embolism and blurred vision. The most common adverse reactions for MMN (observed in ≥5% of subjects) were headache, chest discomfort, muscle spasms, muscular weakness, nausea, oropharyngeal pain, and pain in extremity.
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
The most common adverse reactions observed in ≥5% of clinical study subjects receiving GAMMAGARD LIQUID for CIDP were headache, pyrexia, anemia, leukopenia, neutropenia, illness, blood creatinine increased, dizziness, migraine, somnolence, tremor, nasal dryness, abdominal pain upper, vomiting, chills, nasopharyngitis, and pain in extremity.
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
Treatment of Primary Immunodeficiency (Intravenous)
The safety of GAMMAGARD LIQUID intravenous infusion was evaluated in 61 subjects.
Fifteen adverse reactions in 8 subjects were serious. Of these, two episodes of aseptic meningitis in one subject were deemed possibly related to infusion of GAMMAGARD LIQUID.
There were 400 non-serious adverse reactions. Of these, 217 were rated as mild (transient discomfort that resolves spontaneously or with minimal intervention), 164 were rated as moderate (limited impairment of function and resolves spontaneously or with minimal intervention with no sequelae), and 19 were rated as severe (marked impairment of function or can lead to temporary inability to resume normal life pattern; requires prolonged intervention or results in sequelae). All of the severe non-serious adverse experiences were transient, did not lead to hospitalization, and resolved without complication. One subject withdrew from the study due to a non-serious adverse experience (papular rash).
Adverse reactions with a frequency of ≥5% (defined as adverse events occurring during or within 72 hours of infusion or any causally related event occurring within the study period) are shown in Table 8.
Table 8: Adverse Reactions Occurring in ≥5% of Subjects with PI (Intravenous Administration) Events
By Infusion N (%)
(N=1812 Infusions)
By Subject N (%)
(N=61 Subjects)
Headache
94 (5.2%)
29 (47.5%)
Fatigue
33 (1.8%)
14 (23.0%)
Pyrexia
28 (1.5%)
17 (27.9%)
Nausea
17 (0.9%)
11 (18.0%)
Chills
14 (0.8%)
8 (13.1%)
Rigors
14 (0.8%)
8 (13.1%)
Pain in extremity
13 (0.7%)
7 (11.5%)
Diarrhea
12 (0.7%)
9 (14.8%)
Migraine
12 (0.7%)
4 (6.6%)
Dizziness
11 (0.6%)
8 (13.1%)
Vomiting
11 (0.6%)
9 (14.8%)
Cough
9 (0.5%)
8 (13.1%)
Urticaria
9 (0.5%)
5 (8.2%)
Asthma
7 (0.4%)
6 (9.8%)
Pharyngolaryngeal pain
7 (0.4%)
5 (8.2%)
Rash
6 (0.3%)
4 (6.6%)
Arthralgia
5 (0.3%)
4 (6.6%)
Myalgia
5 (0.3%)
5 (8.2%)
Oedema peripheral
5 (0.3%)
5 (8.2%)
Pruritus
5 (0.3%)
4 (6.6%)
Cardiac murmur
4 (0.2%)
4 (6.6%)
Pooled analysis of 4 short term clinical studies with 106 subjects (total of 854 infusions) showed no differences in the safety profile of GAMMAGARD LIQUID. These short-term studies were designed to stabilize the immune globulin treatment or as a safety follow-up study. They were not designed to study the safety, efficacy and tolerability of GAMMAGARD LIQUID. No additional adverse reactions were reported during the study periods.
Treatment of Primary Immunodeficiency (Subcutaneous)
The safety of GAMMAGARD LIQUID in subcutaneous infusion was evaluated in 47 subjects.
Adverse reactions with a frequency of ≥5% (defined as adverse events occurring during or within 72 hours of infusion or any causally related event occurring within the study period) are shown in Table 9.
Table 9: Adverse Reactions Occurring in ≥5% of Subjects with PI (Subcutaneous Administration) Events
By Infusion N (%)
(N=2294 infusions)
By Subject N (%)
(N=47 Subjects)
Infusion site (local) event
55 (2.4%)
21 (44.7%)
Headache
31 (1.4%)
19 (40.4%)
Fatigue
11 (0.5%)
7 (14.9%)
Heart rate increased
11 (0.5%)
3 (6.4%)
Pyrexia
11 (0.5%)
9 (19.1%)
Abdominal pain upper
9 (0.4%)
5 (10.6%)
Nausea
7 (0.3%)
3 (6.4%)
Vomiting
7 (0.3%)
5 (10.6%)
Asthma
6 (0.3%)
4 (8.5%)
Blood pressure systolic increased
6 (0.3%)
3 (6.4%)
Diarrhea
5 (0.2%)
3 (6.4%)
Ear pain
4 (0.2%)
3 (6.4%)
Aphthous stomatitis
3 (0.1%)
3 (6.4%)
Migraine
3 (0.1%)
3 (6.4%)
Oropharyngeal pain
3 (0.1%)
3 (6.4%)
Pain in extremity
3 (0.1%)
3 (6.4%)
Of the 348 non-serious adverse reactions, 228 were rated as mild (transient discomfort that resolves spontaneously or with minimal intervention), 112 were rated as moderate (limited impairment of function and resolves spontaneously or with minimal intervention with no sequelae), and 8 were rated as severe (marked impairment of function or can lead to temporary inability to resume normal life pattern; requires prolonged intervention or results in sequelae). Neither of the severe adverse reactions required hospitalization or resulted in sequelae.
Local Adverse Reactions:
Local adverse reactions reported as mild (transient discomfort that resolves spontaneously or with minimal intervention) were rash, erythema, edema, hemorrhage, and irritation. Local adverse reactions reported as mild or moderate (limited impairment of function and resolves spontaneously or with minimal intervention with no sequelae) were pain, hematoma, pruritus, and swelling.
One subject withdrew from the study after 10 treatments with GAMMAGARD LIQUID subcutaneous infusion (2.5 months) due to increased fatigue and malaise.
The overall rate of local adverse reactions (excluding infections) during subcutaneous treatment was 2.4% per infusion. In subcutaneous naïve subjects, the incidence of local adverse reactions (N=1757 infusions) was 2.8% (2.2% mild and 0.6% moderate with no severe adverse reactions). In the subjects who were subcutaneous experienced (N=537 infusions), the incidence of local adverse reactions was 1.1% (1.1% mild, and no moderate or severe adverse reactions).
After all subcutaneous doses were adjusted, only one subject did not reach the maximum rate allowed in the protocol for one or more infusions, 20 mL/site/hour if weight was below 40 kg and 30/mL/hour for weight 40 kg and greater. Overall, 70% (31 of 44) of subjects opted for the highest rate for all infusions. No subject limited the infusion rate due to an adverse reaction. Median duration of each weekly infusion was 1.2 hours (range: 0.8-2.3 hours). The rate set on the pump was the rate per site multiplied by the number of sites, with no maximum.
During subcutaneous treatment, 99.8% of infusions were completed without a reduction, interruption, or discontinuation for tolerability reasons. The proportion of subjects who experienced local adverse reactions (excluding infections) was highest immediately following the switch from intravenous to subcutaneous treatment in all age groups. The rate of local adverse reactions per infusion immediately after switching from intravenous to subcutaneous treatment was 4.9% (29/595), decreasing to 1.5% (8/538) by the end of the study and to 1.1% (10/893) in the Study Extension. There was a decrease of local adverse reactions over subsequent subcutaneous infusions.
Eight (17%) subjects experienced a local adverse reaction during the first infusion, but that decreased to 1 (2.2%) for subsequent infusions, ranging from 0 to 4 (8.7%) during the first year of subcutaneous treatment. No subject reported a local adverse reaction from week 53 to end of study at week 68.
Analysis of a short-term follow-up safety study of 10 subjects who were treated with subcutaneous administration of GAMMAGARD LIQUID (total of 218 infusions) showed no differences in the safety profile. The follow-up safety study was not designed to study the safety, efficacy and tolerability of GAMMAGARD LIQUID and no additional adverse reactions were reported during the study period.
Treatment of Multifocal Motor Neuropathy (Intravenous)
The safety of GAMMAGARD LIQUID was evaluated in 44 subjects with MMN who received a total of 983 infusions. Two serious adverse reactions, pulmonary embolism and blurred vision, occurred.
In the study, among the 317 non-serious adverse reactions, 176 were considered ARs. Of these, 126 were mild (transient discomfort that resolves spontaneously or with minimal intervention), 37 were moderate (limited impairment of function and resolves spontaneously or with minimal intervention with no sequelae) and 13 were severe (marked impairment of function or can lead to temporary inability to resume normal life pattern; requires prolonged intervention or results in sequelae).
Adverse reactions with a frequency ≥5% (defined as adverse events occurring during or within 72 hours of infusion or any causally related event occurring within the study period) are shown in Table 10.
Table 10: Adverse Reactions Occurring in ≥5% of MMN Subjects Events
GAMMAGARD LIQUID
Placebo
By Infusion
N (%)
(N=983 Infusions)
By Subject
N (%)
(N=44 Subjects)
By Infusion
N (%)
(N=129 Infusions)
By Subject
N (%)
(N=43 Subjects)
Headache
28 (2.85%)
14 (31.82%)
3 (2.33%)
2 (4.65%)
Chest Discomfort
3 (0.31%)
3 (6.82%)
0 (0.00%)
0 (0.00%)
Muscle Spasms
3 (0.31%)
3 (6.82%)
0 (0.00%)
0 (0.00%)
Muscular weakness
4 (0.41%)
3 (6.82%)
1 (0.78%)
1 (2.33%)
Nausea
28 (2.85%)
3 (6.82%)
2 (1.55%)
1 (2.33%)
Oropharyngeal pain
4 (0.41%)
3 (6.82%)
0 (0.00%)
0 (0.00%)
Pain in extremity
4 (0.41%)
3 (6.82%)
1 (0.78%)
1 (2.33%)
Treatment of Chronic Inflammatory Demyelinating Polyneuropathy (Intravenous)
The safety of GAMMAGARD LIQUID was evaluated in a clinical study of 20 adult subjects with CIDP. A total of 389 infusions of GAMMAGARD LIQUID were administered during the study.
Fourteen out of the 20 subjects reported 60 adverse events; 9 experienced mild events (transient discomfort that resolves spontaneously or with minimal intervention), 3 had moderate events (limited impairment of function and resolves spontaneously or with minimal intervention with no sequelae), and 2 experienced severe events (marked impairment of function or can lead to a temporary inability to resume normal life pattern; requires prolonged intervention or results in sequelae). There were 39 ARs in 13 subjects (65%), and 31 related to GAMMAGARD LIQUID in 11 subjects (55%). One subject (5%) experienced one severe AR (headache) related to GAMMAGARD LIQUID. No AR resulted in early discontinuation or death, and no serious AR was reported.
Adverse reactions with a frequency ≥5% (defined as adverse events occurring during or within 72 hours of infusion or any causally related event occurring within the study period) are shown in Table 11.
Table 11: Adverse Reactions Occurring in ≥5% of CIDP Subjects Events By Infusion N (%)
(N=389 Infusions)By Subject N (%)
(N=20 Subjects)Headache 20 (5.1%) 8 (40.0%) Pyrexia 3 (0.8%) 2 (10.0%) Abdominal pain upper 1 (0.3%) 1 (5.0%) Anemia 1 (0.3%) 1 (5.0%) Blood creatinine increased 1 (0.3%) 1 (5.0%) Chills 2 (0.5%) 1 (5.0%) Dizziness 1 (0.3%) 1 (5.0%) Illness 1 (0.3%) 1 (5.0%) Leukopenia 1 (0.3%) 1 (5.0%) Migraine 1 (0.3%) 1 (5.0%) Nasal Dryness 1 (0.3%) 1 (5.0%) Nasopharyngitis 1 (0.3%) 1 (5.0%) Neutropenia 1 (0.3%) 1 (5.0%) Pain in extremity 1 (0.3%) 1 (5.0%) Somnolence 1 (0.3%) 1 (5.0%) Tremor 1 (0.3%) 1 (5.0%) Vomiting 1 (0.3%) 1 (5.0%) 6.2 Postmarketing Experience
Because postmarketing reporting of adverse reactions is voluntary and from a population of uncertain size, it is not always possible to reliably estimate the frequency of these reactions or establish a causal relationship to product exposure.
Intravenous Adverse Reactions
Blood and Lymphatic System Disorders: Hemolysis
Immune System Disorders: Anaphylactic shock
Nervous System Disorders: Cerebral vascular accident, transient ischemic attack, tremor
Cardiac Disorders: Myocardial infarction
Vascular Disorders: Deep vein thrombosis, hypotension
Respiratory, Thoracic and Mediastinal Disorders: Pulmonary embolism, pulmonary edema
Skin and Subcutaneous Tissue Disorders: Hyperhidrosis
General Disorders and Administration-Site Conditions: Chest pain
Investigations: Coombs direct test positive, oxygen saturation decreased
Injury, Poisoning and Procedural Complications: Transfusion-related acute lung injurySubcutaneous Adverse Reactions
Immune System Disorders: Hypersensitive
Musculoskeletal and Connective Tissue Disorders: Myalgia
General Disorders and Administration-Site Conditions: Chills
In addition to the adverse reactions listed above, the following reactions have been identified for immune globulin products administered intravenously:
Renal and Urinary Disorders: Osmotic nephropathy
Respiratory, Thoracic and Mediastinal Disorders: Cyanosis, hypoxemia, bronchospasm, apnea, Acute Respiratory Distress Syndrome (ARDS)
Integumentary: Bullous dermatitis, epidermolysis, erythema multiforme, Stevens-Johnson Syndrome
Vascular Disorders: Cardiac arrest, vascular collapse
Nervous System Disorders: Coma, seizures, loss of consciousness
Blood and Lymphatic System Disorders: Pancytopenia
Gastrointestinal: Hepatic dysfunction
The adverse reactions listed below have been identified and reported with the use of another immune globulin products administered subcutaneously:
Immune System Disorders: Anaphylactic reaction
Nervous System Disorders: Paresthesia, tremor
Cardiac Disorders: Tachycardia
Vascular Disorders: Hypotension
Respiratory, Thoracic and Mediastinal Disorders: Dyspnea, laryngospasm
General Disorders and Administration-Site Conditions: Chest discomfort, injection site reaction (including induration, warmth) -
7 DRUG INTERACTIONS
Passive transfer of antibodies may transiently impair the immune response to live attenuated virus vaccines such as mumps, rubella and varicella for up to 6 months, and for a year or more to measles (rubeola). Inform the immunizing physician of recent therapy with GAMMAGARD LIQUID so that appropriate precautions can be taken [see Patient Counseling Information (17)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Animal reproduction studies have not been conducted with GAMMAGARD LIQUID. It is not known whether GAMMAGARD LIQUID can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Immune globulins cross the placenta from maternal circulation increasingly after 30 weeks of gestation. GAMMAGARD LIQUID should be given to a pregnant woman only if clearly indicated.
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of GAMMAGARD LIQUID in human milk, its effects on the breastfed infant or its effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for GAMMAGARD LIQUID and any potential adverse effects on the breastfed infant from GAMMAGARD LIQUID or from the underlying maternal condition.
8.4 Pediatric Use
Treatment of Primary Immunodeficiency (PI)
GAMMAGARD LIQUID administered intravenously was evaluated in 15 pediatric subjects with PI (7 subjects aged 2 to <12 years and 8 subjects aged 12 to <16 years) in a multicenter clinical study. GAMMAGARD LIQUID administered subcutaneously was evaluated in 18 pediatric subjects with PI (14 subjects aged 2 to <12 years and 4 subject aged 12 to <16 years) in another multicenter clinical study. The safety and efficacy profiles were similar to adult subjects. No pediatric-specific dose requirements were necessary to achieve the desired serum IgG levels.
Safety and efficacy of GAMMAGARD LIQUID in pediatric patients below the age of 2 have not been established.
Treatment of Multifocal Motor Neuropathy (MMN) and Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
Safety and effectiveness in pediatric patients with MMN and CIDP have not been established.
8.5 Geriatric Use
Treatment of Primary Immunodeficiency (PI)
Limited information is available for the geriatric use of GAMMAGARD LIQUID. GAMMAGARD LIQUID administered intravenously and subcutaneously was evaluated in two PI studies with a total of 8 subjects over the age of 65 years. No differences in safety or efficacy were observed for this group. Monitor patients who are at an increased risk for developing renal failure or thrombotic events. Do not exceed the recommended dose. Infuse at the minimum intravenous infusion rate practicable [see Boxed Warning, Warnings and Precautions (5.2, 5.4) and Dosage and Administration (2.5)].
Treatment of Multifocal Motor Neuropathy (MMN)
GAMMAGARD LIQUID was administered intravenously for treatment of MMN in 5 subjects aged 65 years and above. There was an insufficient number of subjects aged 65 years and above to determine whether they respond differently from younger subjects [see Boxed Warning, Warnings and Precautions (5.2, 5.4) and Dosage and Administration (2.5)].
Treatment of Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
GAMMAGARD LIQUID was administered intravenously for the treatment of CIDP in 5 subjects aged 65 years and above and 15 subjects aged below 65 years. There was an insufficient number of subjects aged 65 years and above to determine whether they respond differently from younger subjects. [see Boxed Warning, Warnings and Precautions (5.2, 5.4) and Dosage and Administration (2.5)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
GAMMAGARD LIQUID is a ready-for-use sterile, liquid preparation of highly purified and concentrated immunoglobulin G (IgG) antibodies. The distribution of the IgG subclasses is similar to that of normal plasma. The Fc and Fab functions are maintained in GAMMAGARD LIQUID. Pre-kallikrein activator activity is not detectable. GAMMAGARD LIQUID contains 100 mg/mL protein. At least 98% of the protein is immune globulin, the average immunoglobulin A (IgA) concentration is 37 µg/mL, and immunoglobulin M is present in trace amounts. GAMMAGARD LIQUID contains a broad spectrum of IgG antibodies against bacterial and viral agents. Glycine (0.25M) serves as a stabilizing and buffering agent. There are no added sugars, sodium or preservatives. The pH is 4.6 to 5.1. The osmolality is 240 to 300 mOsmol/kg, which is similar to physiological osmolality (285 to 295 mOsmol/kg).
GAMMAGARD LIQUID is manufactured from large pools of human plasma. IgG preparations are purified from plasma pools using a modified Cohn-Oncley cold ethanol fractionation process, as well as cation and anion exchange chromatography.
Screening against potentially infectious agents begins with the donor selection process and continues throughout plasma collection and plasma preparation. Each individual plasma donation used in the manufacture of GAMMAGARD LIQUID is collected only at FDA approved blood establishments and is tested by FDA licensed serological tests for Hepatitis B Surface Antigen (HBsAg), and for antibodies to Human Immunodeficiency Virus (HIV-1/HIV-2) and Hepatitis C Virus (HCV) in accordance with U.S. regulatory requirements. As an additional safety measure, mini-pools of the plasma are tested for the presence of HIV-1 and HCV by FDA licensed Nucleic Acid Testing (NAT) and found to be negative.
To further improve the margin of safety, validated virus inactivation/removal steps have been integrated into the manufacturing and formulation processes, namely solvent/detergent (S/D) treatment,10 35 nm nanofiltration, and a low pH incubation at elevated temperature (30 C to 32 C). The S/D process includes treatment with an organic mixture of tri-n-butyl phosphate, octoxynol 9 and polysorbate 80 at 18 C to 25 C for a minimum of 60 minutes. S/D treatment inactivates the lipid-enveloped viruses investigated to below detection limits within minutes.12
In vitro virus spiking studies have been used to validate the capability of the manufacturing process to inactivate and remove viruses. To establish the minimum applicable virus clearance capacity of the manufacturing process, virus clearance studies were performed under extreme conditions (e.g., at minimum S/D concentrations, incubation time and temperature for the S/D treatment).
Virus clearance studies for GAMMAGARD LIQUID performed in accordance with good laboratory practices are summarized in Table 12.
Table 12: Three Dedicated Independent Virus Inactivation/Removal Steps Mean Log10 Reduction Factors* (RFs) For Each Virus and Manufacturing Step Abbreviations: HIV-1, Human Immunodeficiency Virus Type 1; BVDV, Bovine Viral Diarrhea Virus (model for Hepatitis C Virus and other lipid enveloped RNA viruses); WNV, West Nile Virus; PRV, Pseudorabies Virus (model for lipid enveloped DNA viruses, including Hepatitis B Virus); EMCV, Encephalomyocarditis Virus (model for non-lipid enveloped RNA viruses, including Hepatitis A virus [HAV]); MMV, Mice Minute Virus (model for non-lipid enveloped DNA viruses, including B19 virus [B19V]); n.d. (not done), n.a. (not applicable). - *
- For the calculation of these RF data from virus clearance study reports, applicable manufacturing conditions were used. Log10 RFs on the order of 4 or more are considered effective for virus clearance in accordance with the Committee for Medicinal Products for Human Use (CHMP, formerly CPMP) guidelines.
- †
- No RF obtained due to immediate neutralization of HAV by the anti-HAV antibodies present in the product.
Virus type
Enveloped RNA
Enveloped DNA
Non-enveloped
RNANon-enveloped
DNAFamily
Retroviridae
Flaviviridae
Herpesviridae
Picornaviridae
Parvoviridae
Virus
HIV-1
BVDV
WNV
PRV
HAV
EMCV
MMV
SD treatment
>4.5
>6.2
n.a.
>4.8
n.d.
n.d.
n.d
35 nm nanofiltration
>4.5
>5.1
>6.2
>5.6
5.7
1.4
2.0
Low pH treatment
>5.8
>5.5
>6.0
>6.5
n.d.†
>6.3
3.1
Overall log reduction factor (ORF)
>14.8
>16.8
>12.2
>16.9
5.7†
>7.7
5.1
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
GAMMAGARD LIQUID supplies a broad spectrum of opsonizing and neutralizing IgG antibodies against a wide variety of bacterial and viral agents. GAMMAGARD LIQUID also contains a spectrum of antibodies capable of interacting with and altering the activity of cells of the immune system as well as antibodies capable of reacting with cells such as erythrocytes. The role of these antibodies and the mechanism of action of IgG in GAMMAGARD LIQUID have not been fully elucidated.
The mechanism of action of immunoglobulins in the treatment of CIDP in adults has not been fully elucidated but may include immunomodulatory effects.
12.3 Pharmacokinetics
Treatment of Primary Immunodeficiency (Intravenous)
Following intravenous infusion, IGIV products show a biphasic decay curve. The initial (α) phase is characterized by an immediate post-infusion peak in serum IgG and is followed by rapid decay due to equilibration between the plasma and extravascular fluid compartments. The second (β) phase is characterized by a slower and constant rate of decay. The commonly cited "normal" half-life of 18 to 25 days is based on studies in which tiny quantities of radiolabeled IgG are injected into healthy individuals. When radiolabeled IgG was injected into patients with hypogammaglobulinemia or agammaglobulinemia, highly variable half-lives ranging from 12 to 40 days were observed. In other radiolabeled studies, high serum concentrations of IgG and hypermetabolism associated with fever and infection have been seen to coincide with a shortened IgG half-life.
In contrast, pharmacokinetic studies in immunodeficient patients are based on the decline of IgG concentrations following infusion of large quantities of immune globulin. In such studies, investigators have reported uniformly prolonged half-lives of 26 to 35 days. Pharmacokinetic parameters for GAMMAGARD LIQUID were determined from total IgG levels following the fourth infusion in subjects with primary humoral immunodeficiency (N=61) treated intravenously with the product every 3 or 4 weeks according to the regimen used prior to entering the study. Of these, 57 had sufficient pharmacokinetic data to be included in the dataset. The median weight-adjusted dose per subject was 455mg/kg/4 weeks with a range of 262 to 710. Pharmacokinetic parameters are presented in Table 13.
Table 13: Summary of Intravenous Pharmacokinetic Parameters in 57 Subjects Abbreviations: AUC=area under the curve,
Cmax=maximum concentration
Cmin=minimum concentration.Parameter
Median
95% Confidence Interval
Dose of IgG (milligram/kg/4 weeks)
455
Range: 262-710
Elimination Half-Life (T ½ days)
35
(31, 42)
AUC0-21d (milligram∙days/dL)
29139
(27494, 30490)
Cmax (Peak, milligram/dL)
2050
(1980, 2200)
Cmin (Trough, milligram/dL)
1030
(939, 1110)
Incremental recovery (milligram/dL)/(milligram/kg)
2.3
(2.2, 2.6)
Median IgG trough levels were maintained between 960 to 1120mg/dL. These dosing regimens-maintained serum trough IgG levels generally considered adequate to prevent bacterial infections. The elimination half-life of GAMMAGARD LIQUID (35 days) was similar to that reported for other IGIV products.
Treatment of Primary Immunodeficiency (Subcutaneous)
Pharmacokinetic (PK) parameters of subcutaneously administered GAMMAGARD LIQUID were evaluated in subjects with primary immunodeficiency (PI) who were 12 years and older during a clinical study [see Clinical Studies (14)].
Subjects were treated intravenously for 12 weeks with GAMMAGARD LIQUID and then switched to weekly subcutaneous GAMMAGARD LIQUID infusions. Initially, all subjects were treated for a minimum of 12 weeks at a subcutaneous dose that was 130% of the intravenous dose. A comparison of the area under the curve (AUC) for intravenous and subcutaneous infusions done on the first 15 adult subjects determined that the subcutaneous dose required to provide an exposure from subcutaneous administration that was not inferior to the exposure from intravenous administration was 137% of the intravenous dose. Subsequently, all subjects were treated with this dose for 6 weeks after which the dose was individualized for all subjects using IgG trough levels, as described below. After a minimum of 8 weeks at this subcutaneous dose, a PK evaluation was conducted on subjects 12 years of age or older (N=32).
The mean adjusted dose at the end of the study was 137.3% (125.7 to 150.8) of the intravenous dose for subjects 12 years and older, and 141.0% (100.5 to 160.0) for subjects under the age of 12. Thus, a significant dosing difference was not required for children. At this dose adjustment, the geometric mean ratio of the AUC for subcutaneous vs. intravenous GAMMAGARD LIQUID administration was 95.2% (90% confidence limit: 92.3 to 98.2). The peak IgG level occurred 2.9 (1.2 to 3.2) days after subcutaneous administration.
Pharmacokinetic parameters of GAMMAGARD LIQUID administered intravenously versus subcutaneously in the clinical study are shown in Table 14. The mean peak IgG level was lower (1393 ± 289 mg/dL) during subcutaneous treatment than with intravenous treatment (2240 ± 536 mg/dL), consistent with lower weekly doses compared with doses administered every 3 or 4 weeks intravenously. In contrast, the mean trough level was higher when GAMMAGARD LIQUID was given subcutaneously (1202 ± 282 mg/dL) than when it was given intravenously (1050 ± 260 mg/dL), a result of both higher monthly dose and more frequent dosing. The median IgG trough level during intravenous treatment in this clinical study, 1010 mg/dL (95% CI: 940 to 1240), was similar to the median IgG trough level of 1030 mg/dL (95% CI: 939 to 1110) during intravenous treatment as shown in Table 13. By contrast, the median IgG trough level during subcutaneous treatment was higher, at 1260 mg/dL (95% CI: 1060 to 1400).
Table 14: Pharmacokinetic Parameters of Subcutaneously Administered GAMMAGARD LIQUID Compared With GAMMAGARD LIQUID Administered Intravenously Subcutaneous Administration Intravenous Administration Number of Subjects 32 32 Dose * (milligram/kg) Mean ± SD 182.6 ± 48.4 133.2 ± 36.9 Range (min to max) 94.2 to 293.8 62.7 to 195.4 IgG Peak Levels (milligram/dL) Mean ± SD 1393 ± 289 2240 ± 536 Range (min to max) 734 to 1900 1130 to 3610 IgG Trough Levels (milligram/dL) Mean ± SD 1202 ± 282 1050 ± 260 Range (min to max) 621 to 1700 532 to 1460 AUC † (days*milligram/dL) Mean ± SD 9176 ± 1928 9958 ± 2274 Range (min to max) 4695 to 12468 5097 to 13831 Clearance [mL/kg/day] Mean ± SD 2.023 ± 0.528 1.355 ± 0.316 Range (min to max) 1.225 to 3.747 0.880 to 2.340 Treatment of Multifocal Motor Neuropathy (Intravenous)
No full pharmacokinetic study was conducted in subjects with MMN. However, trough levels of IgG were measured in this population (n = 44; five 12 week study parts). The median serum trough level of total IgG over all study parts regardless of dosing intervals and length of infusion cycles, was 1640 (95% confidence interval: 1570 to 1710)mg/dL. During placebo administration, the median trough level was 1235 (95% CI: 1060 to 1360)mg/dL. The relationship between serum IgG concentration and efficacy was not assessed.
Treatment of Chronic Inflammatory Demyelinating Polyneuropathy (Intravenous)
The full pharmacokinetic profile of GAMMAGARD LIQUID was not evaluated but serum trough IgG levels were measured in subjects with CIDP following administrations of GAMMAGARD LIQUID in the clinical study [see Clinical Studies (14)]. In 16 subjects who had previously received placebo in a preceding study and started administration of GAMMAGARD LIQUID after relapse, the median (range, number of subjects) serum trough IgG levels at baseline, Week 13, and Week 25 were 1220 (449 to 2220, N=16) mg/dL, 1810 (590 to 3200, N=13) mg/dL, and 1615 (712 to 3480, N=14) mg/dL, respectively.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been conducted to evaluate the carcinogenic potential of GAMMAGARD LIQUID or its effect on fertility.
An in vitro mutagenicity test was performed with GAMMAGARD LIQUID. No evidence of mutagenicity was observed.
-
14 CLINICAL STUDIES
Treatment of Primary Immunodeficiency (Intravenous)
Intravenous use of GAMMAGARD LIQUID is supported by a study in subjects (N=61) who were treated with 300 to 600 mg/kg every 21 to 28 days for 12 months. The age range of subjects was 6 to 72 years, with 54% female and 46% male, and 93% Caucasian, 5% African American, and 2% Asian. Three subjects were excluded from the per-protocol analysis due to non-study product related reasons. The annualized rate of prespecified acute serious bacterial infections, i.e., the mean number of prespecified acute serious bacterial infections per subject per year, was studied (see Table 15).
Table 15: Summary of Validated Acute Serious Bacterial Infections for the Per-Protocol Analysis Number of Events
Validated Infections*
Bacteremia / Sepsis
0
Bacterial Meningitis
0
Osteomyelitis / Septic Arthritis
0
Bacterial Pneumonia
0
Visceral Abscess
0
Total
0
Hospitalizations Secondary to Infection
0
Mean Number of Validated Infections per Subject per Year
0
p-value†
p < 0.0001
95% Confidence Interval†
(0.000, 0.064)
The annualized rate of other prespecified validated bacterial infections (see Table 16), and the number of hospitalizations secondary to all validated infectious complications also were studied (see Table 15 and Table 16).
Table 16: Summary of Validated Other Bacterial Infections - *
- Other bacterial infections that met specific diagnostic requirements
Number of Events
Validated Infections *
Urinary Tract Infection
1
Gastroenteritis
1
Lower Respiratory Tract Infection: Tracheobronchitis, Bronchiolitis (Without Evidence of Pneumonia)
0
Lower Respiratory Tract Infection:
Other Infections (e.g., Lung Abscess, Empyema)0
Otitis Media
2
Total
4
Hospitalizations Secondary to Infection
0
Mean Number of Validated Infections per Subject per Year
0.07
95% Confidence Interval
(0.018, 0.168)
None of the 61 treated subjects was positive for HCV, HIV-1, and HIV-2 and HBV prior to study entry and none converted from negative to positive during the 12-month period.
Treatment of Primary Immunodeficiency (Subcutaneous)
A prospective, open-label, non-controlled, multicenter study was conducted in the U.S. to determine the efficacy, tolerability and PK of GAMMAGARD LIQUID subcutaneous infusion in adult and pediatric subjects (N=49) with PI. All subjects were treated for 12 weeks with GAMMAGARD LIQUID intravenous infusion every 3 or 4 weeks. Subjects who were on intravenous treatment prior to entering the study were switched to GAMMAGARD LIQUID at the same dose and frequency. Subjects who were receiving subcutaneous immune globulin were switched to GAMMAGARD LIQUID at the intravenous dose they had received prior to switching to subcutaneous treatment. A PK analysis was performed at the end of the intravenous period in all subjects aged 12 years and older.
One week after the last intravenous infusion, each subject began subcutaneous treatment with GAMMAGARD LIQUID at 130% of the weekly equivalent of the intravenous dose for a minimum of 12 weeks. PK data from the first 15 adult subjects were used to determine the dose required to ensure that the IgG exposure with subcutaneous treatment was not inferior to that with intravenous treatment. The median dose determined from these subjects was 137% of the intravenous dose, and subsequently all subjects were treated for a minimum of 6 weeks at this dose. After 6 subcutaneous infusions, a trough IgG level was obtained and used to individually adapt the subcutaneous dose of GAMMAGARD LIQUID to compensate for individual variation from the mean value of 137% [see Pharmacokinetics (12.3) and Dosage and Administration (2.1)].
All subjects received a minimum of 12 infusions at this individually adapted dose and continued to receive subcutaneous treatment with GAMMAGARD LIQUID until the last subject completed the study. Subjects (N=47) were treated with 2,294 subcutaneous infusions of GAMMAGARD LIQUID: 4 subjects treated for up to 29 weeks, 17 subjects for 30 to 52 weeks, and 26 subjects for 53 weeks or longer. Two subjects that completed the intravenous treatment part of the study did not continue to the subcutaneous treatment part of the study. The median duration of subcutaneous treatment was 379 days (range: 57 to 477 days).
Efficacy was determined throughout the entire subcutaneous phase. There were 31 adults aged 16 years or older, 4 adolescents aged 12 to <16 years, and 14 children aged 2 to <12 years. The volume of GAMMAGARD LIQUID infused was 30 mL per site for subjects weighing 40 kg and greater, and 20 mL per site for those weighing less than 40 kg. The total weekly dose was divided by those values to determine the number of sites.
Mean weekly subcutaneous doses ranged from 181.9 mg/kg to 190.7 mg/kg (at 130% to 137% of the intravenous dose). In the study, the number of infusion sites per infusion was dependent on the dose of IgG and ranged from 2 to 10. In 75% of infusions, the number of infusion sites was 5 or fewer.
There were 3 serious validated bacterial infections, all bacterial pneumonia. None of these subjects required hospitalization to treat their infection. The annual rate of acute serious bacterial infections while on GAMMAGARD LIQUID subcutaneous treatment was 0.067, with an upper 99% confidence limit of 0.133, which is lower than the minimal goal of achieving a rate of <1 bacterial infection per patient-year.
Table 17 presents a summary of infections and associated events for subjects during subcutaneous treatment with GAMMAGARD LIQUID. The annual rate of any infection in this study during subcutaneous treatment, including viral and fungal infections, was 4.1 infections per subject per year.
Table 17: Summary of Infections and Associated Events - *
- Included systemic and topical antibacterial, anti-fungal, anti-viral, and anti-protozoal antimicrobials
Number of subjects (efficacy phase)
Total number of subject years
Annual rate of any infections
47
44
4.1 (95% CI 3.2 to 5.1)
infections/subject year
Antibiotic use* (prophylaxis or treatment)
Number of subjects (%)
Annual rate
40 (85.1%)
50.2 (95% CI 33.4 to 71.9)
days/subject year
Days out of work/school/day care or unable to perform normal activities
Number of subjects (%)
Annual rate
25 (53.2%)
4.0 (95% CI 2.5 to 6.1)
days/subject year
Hospitalizations due to infections
Number of subjects (%)
Annual rate
0 (0.0%)
0.0 (95% CI 0.0 to 0.1)
days/subject year
Treatment of Multifocal Motor Neuropathy
A randomized, double-blind, placebo controlled, cross-over withdrawal study was conducted to evaluate the efficacy and safety/tolerability of GAMMAGARD LIQUID in adult subjects (N=44) with MMN.12 The study examined grip strength in the more affected hand11 (measured with dynamometer), and Guy's Neurological Disability Scale (GNDS) [upper limb part 6 subsection].13 Study subjects were on a regimen of licensed immunoglobulin (existing maintenance dose ranging from 0.5 to 2.0 grams/kg/month) prior to enrollment and thus, the results cannot be generalized to naïve patients.
The study comprised of five study periods, each lasting 12 weeks: 3 stabilization phases, one randomized withdrawal phase and one cross-over phase. Open-label GAMMAGARD LIQUID was administered at the beginning (study period 1) and at the end of the study (study period 5) for clinical stabilization, and between the double-blinded periods to prevent carry-over effect (study period 3). If, during either of the double-blinded treatment periods, the subject's upper limb function involving the affected muscles deteriorated such that the subject had difficulty completing daily activities or experienced a decline in grip strength of ≥50% in the more affected hand, the subject was switched directly to the next stabilization phase of open-label GAMMAGARD LIQUID ("accelerated switch") without breaking the blind.
All subjects were treated for 12 weeks with open-label GAMMAGARD LIQUID during initial stabilization (study period 1). Each subject was then randomized in a double-blind manner to continuation of GAMMAGARD LIQUID or withdrawal of GAMMAGARD LIQUID and replacement by placebo for 12 weeks (study period 2); subjects who did not tolerate treatment were immediately transitioned to open label GAMMAGARD LIQUID. After infusion of open-label GAMMAGARD LIQUID for 12 weeks (study period 3), subjects crossed-over to receive placebo or GAMMAGARD LIQUID for 12 weeks (study period 4). No subject was allowed to experience placebo more than one time during the study. At study end, subjects were treated with open-label GAMMAGARD LIQUID for 12 weeks (study period 5).
Overall, 69% (n=29) of subjects required an accelerated switch to open-label treatment with GAMMAGARD LIQUID during the placebo period due to functional deterioration, but did not switch when receiving GAMMAGARD LIQUID. The median number of treatment days using GAMMAGARD LIQUID was 84 and the median number of days using placebo was 28. One subject (2.4%) switched to open‑label treatment during blinded GAMMAGARD LIQUID cross-over period 1, but did not switch during placebo administration (p <0.001).
Forty-four subjects were evaluated to demonstrate effectiveness of GAMMAGARD LIQUID to improve or maintain muscle strength and functional ability in patients with MMN.
Statistical significance (p<0.001) favoring GAMMAGARD LIQUID over placebo was demonstrated by a substantially lower decline from baseline (22.30%; 95% CI: 9.92% to 34.67%) in mean grip strength in the more affected hand following treatment (see Table 18). The difference in relative change for GAMMAGARD LIQUID and placebo of 22.94% (95% CI: 10.69 to 35.19).
Table 18: Relative Change in Grip Strength in the More Affected Hand during Cross-over Period (ANoVA) (mIntent-to-Treat Dataset) No. of subjects (N=41) - *
- A single subject in sequence 2, who was considered an outlier, was excluded from analysis
Statistics
Sequence 1
Sequence 2
Difference
GAMMAGARD LIQUID
Placebo
Placebo
GAMMAGARD LIQUID
(GAMMAGARD LIQUID - Placebo)
N
22
22
19
20*
41
Mean (SD)
-16.36 (32.84)
-30.52 (29.68)
-29.19
(39.95)1.46
(10.72)22.30
(39.21)Median
-3.90
-27.00
-25.03
-0.11
26.6
Guy's Neurological Disability Scores (GNDS)12 for the upper limbs, reflecting both fine motor skills and proximal strength, showed a significant difference in efficacy between GAMMAGARD LIQUID and placebo at the 2.5% level in favor of GAMMAGARD LIQUID. GNDS is a patient orientated clinical disability scale designed for multiple sclerosis and is considered appropriate for other neurological disorders.
As determined by GNDS scores for the upper limbs, 35.7% of subjects deteriorated while receiving placebo but not during treatment with GAMMAGARD LIQUID, whereas 11.9% of subjects deteriorated during GAMMAGARD LIQUID but not during the placebo period. This difference was statistically significant (p=0.021) (see Table 19). Overall, 4.8% of subjects showed deterioration with both placebo and GAMMAGARD LIQUID, while 47.6% showed no deterioration using either.
Table 19: McNemar’s Test for Subjects with Deterioration in Guy’s Neurological Disability Score (Intent-to-Treat Dataset) No. of subjects (N=42) Deterioration on Placebo
15 (35.7%)
Deterioration on GAMMAGARD LIQUID
5 (11.9%)
Deterioration on both
2 (4.8%)
No deterioration
20 (47.6%)
When data from both treatment sequences were combined, a relative decline of ≥30% in grip strength in the more affected hand occurred in 42.9% of subjects during the placebo period, but not during treatment with GAMMAGARD LIQUID, whereas 4.8% of subjects experienced a ≥30% decline during treatment with GAMMAGARD LIQUID, but not during placebo. A relative decline of ≥30% in grip strength in the less affected hand occurred in 31.0% of subjects during the placebo period, but not during treatment with GAMMAGARD LIQUID. No subject experienced a ≥30% decline during treatment with GAMMAGARD LIQUID.
The Overall Disability Sum Score (ODSS) changed by -7.14% during placebo (indicating worsening of disability) and by -1.11% (indicating minimal change in disability) during treatment with GAMMAGARD LIQUID. For this specific analysis of ODSS, lower scores represented more disability.
With the dominant hand, subjects required 17% longer to complete the 9-hole peg test (a measure of dexterity) at the end of the placebo period, compared with baseline. By contrast, at the end of the GAMMAGARD LIQUID treatment period, subjects required 1.2% longer to complete the 9-hole peg test for the dominant hand compared with baseline. With the non-dominant hand, subjects required 33% longer to complete the 9-hole peg test at the end of the placebo period and 6.7% longer at the end of the GAMMAGARD LIQUID treatment period, compared with baseline.
Compared with baseline, assessment by subjects of physical functioning, as measured by visual analog scale (VAS) showed a mean change of 290% during placebo compared with baseline. Assessment by subjects of physical functioning showed a mean change of 73% during GAMMAGARD LIQUID treatment. Higher visual analog scale scores represent more severe disability.
Treatment of Chronic Inflammatory Demyelinating Polyneuropathy
In a prospective, open-label, single-arm, multicenter clinical study, a total of 18 subjects who developed a relapse in Epoch 1 and received GAMMAGARD LIQUID in Epoch 2 were included in efficacy analyses. GAMMAGARD LIQUID was administered at an induction dose of 2 g/kg body weight, followed by maintenance infusions at every 3 weeks for a period of 6 months. The dose of GAMMAGARD LIQUID treatment could be adjusted at the investigator's discretion. Adjustments to the dosing interval of every 3 weeks were not allowed. All subjects completed the study. All dosed subjects were analyzed for efficacy and safety.
Efficacy in Epoch 2 was based on responder rate, where a responder was defined as a subject who demonstrated an improvement of functional disability, indicated by at least a 1 point decrease in the adjusted Inflammatory Neuropathy Cause and Treatment (INCAT) disability score at the completion of the IV treatment period (6 months) or the last study visit of the IV treatment period, relative to pre-IV treatment baseline. The responder rate was 94.4% (N=18, 95% CI: 74.2% to 99.0%). The adjusted INCAT score returned back to baseline values prior to joining the study in 17 of the 18 subjects (94.4%) at 6 months. All subjects (N=18) had improvement in functional ability. Functional ability was a composite measure based on meeting any of the following criteria: decrease of ≥1 point in the adjusted INCAT disability score, increase of ≥8 kPa in hand grip strength in the more affected hand, or increase of ≥4 points in raw summed RODs score.
The mean adjusted INCAT score showed an improvement by 2.1 points. Medical Research Council (MRC) sum score improved by a mean of 5.4 points. The mean change in centile Rasch-built Overall Disability Scale (RODS) score was 15.0 points. Grip strength improved by a mean of 13.8 kPa in the more affected hand and 9.8 kPa in the less affected hand.
-
15 REFERENCES
- 1.
- Orange JS, Hossny EM, Weiler CR, Ballow M, Berger M, Bonilla FA, Buckley R, Chinen J, El-Gamal Y, Mazer BD, Nelson Jr. RP, Patel DD, Secord E, Sorenson RU, Wasserman RL, Cunningham-Rundles C, Use of Intravenous Immunoglobulin in Human Disease: A Review of Evidence by Members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma, and Immunology. J Allergy Clin Immunol 2006; 117:S525-53.
- 2.
- Bonilla FA, Bernstein IL, Khan DA, Ballas ZK, Chinen J, Frank MM, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005; 94(suppl 1):S1-63.
- 3.
- Pierce LR, Jain N. Risks associated with the use of intravenous immunoglobulin. Transfusion Med Rev. 2003;17:241-251.
- 4.
- Katz U, Sheonfeld Y. Review: intravenous immunoglobulin therapy and thromboembolic complications. Lupus 2005;14:802-8.
- 5.
- Wilson JR, Bhoopalam N, Fisher M. Hemolytic anemia associated with intravenous immunoglobulin. Muscle Nerve 1997;20:1142-1145.
- 6.
- Kessary-Shoham H, Levy Y, Shoenfeld Y, Lorber M, Gershon H. In vivo administration of intravenous immunoglobulin (IVIg) can lead to enhanced erythrocyte sequestration. J Autoimmun 1999;13:129-135.
- 7.
- Daw Z, Padmore R, Neurath D, Cober N, Tokessy M, Desjardins D, et al. Hemolytic transfusion reactions after administration of intravenous immune (gamma) globulin: a case series analysis. Transfusion. 2008; 48:1598-601.
- 8.
- Copelan EA, Strohm PL, Kennedy MS, Tuschka PJ, Hemolysis following intravenous immune globuline therapy. Transfusion 1986;26:410-412.
- 9.
- Kahwaji J, et al.; Acute hemolysis after High-Dose Intravenous Immunoglobin Therapy in Highly HLA Sensitized Patients. Clin J Am Soc Nephrol; 2009 (4):1993-97.
- 10.
- Kreil TR, Berting A, Kistner O, Kindermann J. West Nile virus and the safety of plasma derivatives: verification of high safety margins, and the validity of predictions based on model virus data. Transfusion 2003;43:1023-1028.
- 11.
- Shechtman O, Gestewitz L, Kimble C. Reliability and validity of the DynEx dynamometer. J. Hand Ther. 2005;18:339-347.
- 12.
- Hahn A., et al.; A controlled trial of intravenous immunoglobulin in multifocal motor neurophathy. Journal of the Peripheral Nervous System 2013; 18:321-330
- 13.
- Sharrack B, Hughes RA. The Guy's Neurological Disability Scale (GNDS): a new disability measure for multiple sclerosis. Mult. Scler. 1999;5:223-233.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
GAMMAGARD LIQUID is supplied in single use bottles containing the labeled amount of functionally active IgG. The packaging of this product is not made with natural rubber latex.
The following presentations of GAMMAGARD LIQUID are available:
NDC Number
Volume
Grams Protein
0944-2700-02
10 mL
1.0
0944-2700-03
25 mL
2.5
0944-2700-04
50 mL
5.0
0944-2700-05
100 mL
10.0
0944-2700-06
200 mL
20.0
0944-2700-07
300 mL
30.0
- ▪
- Do not freeze.
- ▪
- Store GAMMAGARD LIQUID in the refrigerator or at room temperature.
- ▪
- Refrigeration: 2° to 8°C [36° to 46°F] for up to 36 months.
- ▪
- Room Temperature: up to 25°C [77°F] for up to 24 months.
- ▪
- Expiration dates for both storage conditions are printed on the outer carton and vial label.
- ▪
- Do not use past the applicable expiration date.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Inform patients to immediately report the following signs and symptoms to their healthcare provider:
- ▪
- Decreased urine output, sudden weight gain, fluid retention/edema, and/or shortness of breath [see Warnings and Precautions (5.2)].
- ▪
- Instruct patients to immediately report symptoms of thrombosis. These symptoms may include pain and/or swelling of an arm or leg with warmth over the affected area, discoloration of an arm or leg, unexplained shortness of breath, chest pain or discomfort that worsens on deep breathing, unexplained rapid pulse, numbness or weakness on one side of the body [see Warnings and Precautions (5.4)].
- ▪
- Severe headache, neck stiffness, drowsiness, fever, sensitivity to light, painful eye movements, nausea, and vomiting [see Warnings and Precautions (5.5)].
- ▪
- Increased heart rate, fatigue, yellowing of the skin or eyes, and dark-colored urine [see Warnings and Precautions (5.6)].
- ▪
- Trouble breathing, chest pain, blue lips or extremities, or fever that can occur 1 to 6 hours after an infusion of GAMMAGARD LIQUID [see Warnings and Precautions (5.7)].
Prior to starting GAMMAGARD LIQUID ask about a history of IgA deficiency, allergic reactions to immune globulin or other blood products. Patients with a history of allergic reactions should not be treated subcutaneously at home until several treatments have been administered and tolerated under medical supervision.
Inform patients that GAMMAGARD LIQUID is made from human plasma and may contain infectious agents that can cause disease (e.g., viruses and, theoretically, the vCJD agent). The risk of GAMMAGARD LIQUID transmitting an infectious agent has been reduced by screening plasma donors for prior exposure, testing donated plasma, and inactivating or removing certain viruses during manufacturing. Patients should report any symptoms that concern them which might be caused by virus infections [see Warnings and Precautions (5.8)].
Inform patients that GAMMAGARD LIQUID can interfere with their immune response to live viral vaccines such as measles, mumps, rubella and varicella, and instruct patients to notify their healthcare professional of this potential interaction when they are receiving vaccinations [see Drug Interactions (7)].
Subcutaneous (SC) Administration Only
Self-administration – If self-administration is deemed to be appropriate by the physician, clear instructions and training on subcutaneous infusion should be given to the patient/caregiver, and the demonstration of their ability to independently administer subcutaneous infusions should be documented.
- ▪
- Ensure the patient understands the importance of consistent weekly subcutaneous infusion to maintain appropriate steady IgG levels.
- ▪
- Instruct the patient to keep a treatment diary/logbook. This diary/logbook should include information about each infusion such as, the time, date, dose, lot number(s) and any reactions.
- ▪
- Inform the patient that mild to moderate local infusion-site reactions (e.g., swelling and redness) are a common side effect of subcutaneous treatment, but to contact their healthcare professional if a local reaction increases in severity or persists for more than a few days.
Manufactured by:
Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
U.S. License No. 1898GAMMAGARD LIQUID® and GAMMAGARD® are registered trademarks of Baxalta Incorporated, a Takeda company.
TAKEDA® and the TAKEDA Logo® are registered trademarks of Takeda Pharmaceutical Company Limited.Revised: 1/2024
GLQ352 -
PATIENT PACKAGE INSERT
Patient Information
GAMMAGARD LIQUID
Immune Globulin Infusion (Human) 10%
For Intravenous and Subcutaneous AdministrationInformation for Patients
The following summarizes important information about GAMMAGARD LIQUID. Please read it carefully before using this medicine. This information does not take the place of talking with your healthcare provider, and it does not include all of the important information about GAMMAGARD LIQUID. If you have any questions after reading this, ask your healthcare provider.
What is the most important information I need to know about GAMMAGARD LIQUID?
GAMMAGARD LIQUID can cause the following serious reactions:
- Severe allergic reactions causing difficulty in breathing or skin rashes
- Decreased kidney function or kidney failure
- Blood clots in the heart, brain, lungs or elsewhere in the body
- Severe headache, drowsiness, fever, painful eye movements, or nausea and vomiting
- Dark colored urine, swelling, fatigue, or difficulty breathing
What is GAMMAGARD LIQUID?
GAMMAGARD LIQUID is a ready-to-use, liquid medicine that contains immunoglobulin G (IgG) antibodies, which protect the body against infection. GAMMAGARD LIQUID is used to treat patients with primary immunodeficiency diseases (PI), patients with multifocal motor neuropathy (MMN) and patients with chronic inflammatory demyelinating polyneuropathy (CIDP).
There are many forms of PI. The most common types of PI result in an inability to make a very important type of protein called antibodies, which help the body fight off infections from bacteria or viruses. GAMMAGARD LIQUID is made from human plasma that is donated by healthy people. GAMMAGARD LIQUID contains antibodies collected from these healthy people that replace the missing antibodies in PI patients.
MMN is a rare disease that causes muscle weakness that worsens over time. It affects the strength of the lower parts of arms and hands more than the legs, usually without affecting the touch sensation.
CIDP is a rare disease. People who have CIDP are believed to have an autoimmune disease in which the body's immune system targets the nerves, leading to muscle weakness and numbness, usually in the arms and legs. IgG is thought to reduce the damage to the nerve and assist in defending the nerve from harm.
Who should not use GAMMAGARD LIQUID?
Do not use GAMMAGARD LIQUID if you have a known history of a severe allergic reaction to immune globulin or other blood products. If you have such a history, discuss this with your healthcare provider to determine if GAMMAGARD LIQUID can be given to you. Tell your healthcare provider if you have a condition called selective (or severe) immunoglobulin A (IgA) deficiency.
How should I use GAMMAGARD LIQUID?
GAMMAGARD LIQUID is given into a vein (intravenously) or under the skin (subcutaneously). For patients with PI, infusions into the vein are usually given every 3 or 4 weeks whereas infusions under the skin are given every week. For patients with MMN, infusions are given into a vein every 2 to 4 weeks as ordered by your physician. You and your healthcare provider will decide which way is best for you. Most of the time infusions under the skin are given at home by patients or caregivers. Although it is possible to give yourself infusions into the vein at home they are more often given in a hospital or infusion center by a nurse.
Instructions for giving GAMMAGARD LIQUID under the skin (subcutaneously) are provided in the Instructions for Use brochure. Only use GAMMAGARD LIQUID by yourself after you have been instructed by your healthcare provider.
What should I avoid while taking GAMMAGARD LIQUID?
GAMMAGARD LIQUID can make vaccines (like measles/mumps/rubella or chickenpox vaccines) not work as well for you. Before you get any vaccines, tell your healthcare provider that you take GAMMAGARD LIQUID.
Tell your healthcare provider if you are pregnant, or plan to become pregnant, or if you are nursing.
What are the possible or reasonably likely side effects of GAMMAGARD LIQUID?
The following one or more possible reactions may occur at the site of infusion. These generally go away within a few hours and are less likely after the first few infusions.
- Mild or moderate pain
- Swelling
- Itching
- Redness
- Bruising
- Warmth
During the infusion of GAMMAGARD LIQUID, look out for the first signs of the following common side effects:
- Headache
- Migraine
- Fever
- Fatigue
- Itching
- Rash/Hives
- Cough
- Chest pain/tightness
- Chills/Shaking chills
- Dizziness
- Nausea/Vomiting
- Faster Heart Rate
- Upper Abdominal Pain
- Increased Blood Pressure
- Muscle cramps
- Sore throat
- Anemia
- Illness
- Somnolence
- Tremor
- Nasal dryness
- Nasopharyngitis
- Pain in extremity
If any of the following problems occur after starting treatment with GAMMAGARD LIQUID, stop the infusion immediately and contact your healthcare provider or call emergency services. These could be signs of a serious problem.
- ▪
- Hives, swelling in the mouth or throat, itching, trouble breathing, wheezing, fainting or dizziness. These could be signs of a serious allergic reaction.
- ▪
- Bad headache with nausea, vomiting, stiff neck, fever, and sensitivity to light. These could be signs of irritation of the lining around your brain.
- ▪
- Reduced urination, sudden weight gain, or swelling in your legs. These could be signs of a kidney problem.
- ▪
- Pain, swelling, warmth, redness, or a lump in your legs or arms. These could be signs of a blood clot.
- ▪
- Brown or red urine, fast heart rate, yellow skin or eyes. These could be signs of a liver problem or a blood problem.
- ▪
- Chest pain or trouble breathing, or blue lips or extremities. These could be signs of a serious heart or lung problem.
- ▪
- Fever over 100°F. This could be a sign of an infection.
These are not all of the possible side effects with GAMMAGARD LIQUID. You can ask your healthcare provider for physician's information leaflet. Tell your healthcare provider about any side effect that bothers you or that does not go away.
Whenever giving yourself treatments at home, you should have another responsible person present to help treat side effects or get help if you have a serious adverse reaction occur. Ask your healthcare provider whether you should have rescue medications, such as antihistamines or epinephrine.
How do I store GAMMAGARD LIQUID?
Store vials in their original boxes to protect from light. Do not freeze GAMMAGARD LIQUID.
You can store GAMMAGARD LIQUID in the refrigerator or at room temperature. The maximum storage time for GAMMAGARD LIQUID depends on the storage temperature you choose.
In the Refrigerator: at 2 to 8°C (36° to 46°F) for up to 36 months.
Room Temperature: up to 25°C (77°F) for up to 24 months.
The refrigerator and room temperature expiration dates are printed on the vial labels and the box. Always check the expiration date. You should not use the product after the expiration date.
Note: If you remove GAMMAGARD LIQUID from the refrigerator and store it at room temperature, do not refrigerate again.
Resources at Takeda Available to the Patients:
For more information on patient resources, education, or insurance assistance please visit www.immunedisease.com.
Detailed Instructions for Subcutaneous Administration for Patients with PI ONLY
Do not begin subcutaneous treatment with GAMMAGARD LIQUID until you have received instructions as detailed above and are comfortable that you can perform all the steps on your own.
Manufactured by:
Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
U.S. License No. 1898
GAMMAGARD LIQUID® and GAMMAGARD® are registered trademarks of Baxalta Incorporated, a Takeda company.
TAKEDA® and the TAKEDA Logo® are registered trademarks of Takeda Pharmaceutical Company Limited.Revised: 1/2024
GLQ352 -
PRINCIPAL DISPLAY PANEL - 10 mL Bottle Label
NDC 0944-2700-08
Single-dose containerImmune Globulin Infusion
(Human) 10%
GAMMAGARD LIQUID1g
10mLSolution for Infusion
For Intravenous or Subcutaneous Use.
Not made with natural rubber latex.Rx Only
Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
U.S. License No. 1898LE-07-54197
Takeda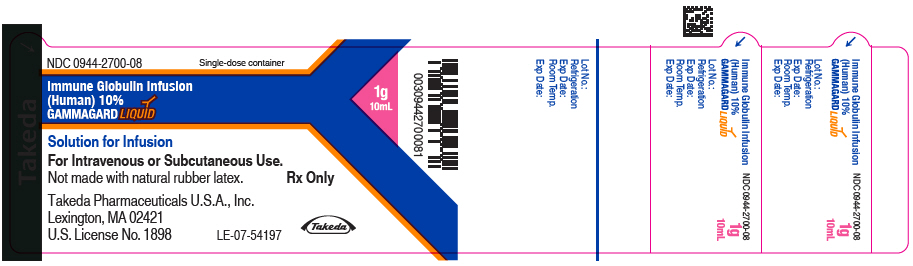
- PRINCIPAL DISPLAY PANEL - 10 mL Bottle Carton
- PRINCIPAL DISPLAY PANEL - 25 mL Bottle Label
- PRINCIPAL DISPLAY PANEL - 25 mL Bottle Carton
-
PRINCIPAL DISPLAY PANEL - 50 mL Bottle Label
NDC 0944-2700-10
Single-dose containerImmune Globulin Infusion
(Human) 10%
GAMMAGARD LIQUID5g
50mLSolution for Infusion
For Intravenous or Subcutaneous Administration.
Not made with natural rubber latex.
Refrigeration: 2°-8°C (36°F-46°F). Do not freeze.
Room temperature: up to 25°C (77°F).Rx Only
Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
U.S. License No. 1898LE-07-54204
Takeda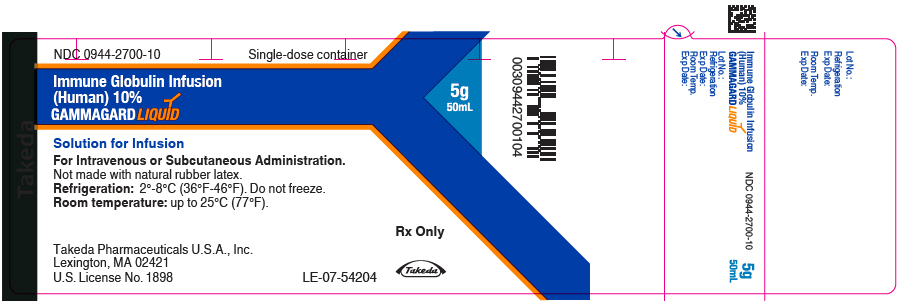
- PRINCIPAL DISPLAY PANEL - 50 mL Bottle Carton
-
PRINCIPAL DISPLAY PANEL - 100 mL Bottle Label
NDC 0944-2700-11
Single-dose containerImmune Globulin Infusion
(Human) 10%
GAMMAGARD LIQUID10g
100mLSolution for Infusion
For Intravenous or Subcutaneous Administration.
Not made with natural rubber latex.
Refrigeration: 2°-8°C (36°F-46°F). Do not freeze.
Room temperature: up to 25°C (77°F).Rx Only
Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
U.S. License No. 1898LE-07-54206
Takeda
- PRINCIPAL DISPLAY PANEL - 100 mL Bottle Carton
-
PRINCIPAL DISPLAY PANEL - 200 mL Bottle Label
NDC 0944-2700-12
Single-dose containerImmune Globulin Infusion
(Human) 10%
GAMMAGARD LIQUID20g
200mLSolution for Infusion
For Intravenous or Subcutaneous Administration.
Not made with natural rubber latex.
Refrigeration: 2°-8°C (36°F-46°F). Do not freeze.
Room temperature: up to 25°C (77°F).Rx Only
Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
U.S. License No. 1898LE-07-54208
Takeda
- PRINCIPAL DISPLAY PANEL - 200 mL Bottle Carton
-
PRINCIPAL DISPLAY PANEL - 300 mL Bottle Label
NDC 0944-2700-13
Single-dose containerImmune Globulin Infusion
(Human) 10%
GAMMAGARD LIQUID30g
300mLSolution for Infusion
For Intravenous or Subcutaneous Administration.
Not made with natural rubber latex.
Refrigeration: 2°-8°C (36°F-46°F). Do not freeze.
Room temperature: up to 25°C (77°F).Rx Only
LE-07-54210
Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
U.S. License No. 1898Takeda
- PRINCIPAL DISPLAY PANEL - 300 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
GAMMAGARD LIQUID
immune globulin infusion (human) injection, solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0944-2700 Route of Administration INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN IMMUNOGLOBULIN G (UNII: 66Y330CJHS) (HUMAN IMMUNOGLOBULIN G - UNII:66Y330CJHS) HUMAN IMMUNOGLOBULIN G 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCINE (UNII: TE7660XO1C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-2700-02 1 in 1 CARTON 1 NDC:0944-2700-08 10 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 2 NDC:0944-2700-03 1 in 1 CARTON 2 NDC:0944-2700-09 25 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 3 NDC:0944-2700-04 1 in 1 CARTON 3 NDC:0944-2700-10 50 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 4 NDC:0944-2700-05 1 in 1 CARTON 4 NDC:0944-2700-11 100 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 5 NDC:0944-2700-06 1 in 1 CARTON 5 NDC:0944-2700-12 200 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 6 NDC:0944-2700-07 1 in 1 CARTON 6 NDC:0944-2700-13 300 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125105 04/27/2005 Labeler - Takeda Pharmaceuticals America, Inc. (039997266) Establishment Name Address ID/FEI Business Operations Baxalta Belgium Manufacturing SA 370634700 MANUFACTURE(0944-2700) , ANALYSIS(0944-2700) , API MANUFACTURE(0944-2700) , LABEL(0944-2700) , PACK(0944-2700) Establishment Name Address ID/FEI Business Operations Baxtalta US Inc 079887650 ANALYSIS(0944-2700) , PACK(0944-2700) , MANUFACTURE(0944-2700)