Label: GANCICLOVIR capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 63304-636-10, 63304-636-28, 63304-636-60, 63304-636-90, view more63304-637-05, 63304-637-28, 63304-637-60, 63304-637-90 - Packager: Ranbaxy Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 20, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING
THE CLINICAL TOXICITY OF GANCICLOVIR INCLUDES GRANULOCYTOPENIA, ANEMIA AND THROMBOCYTOPENIA. IN ANIMAL STUDIES GANCICLOVIR WAS CARCINOGENIC, TERATOGENIC AND CAUSED ASPERMATOGENESIS.
GANCICLOVIR CAPSULES ARE INDICATED ONLY FOR PREVENTION OF CMV DISEASE IN PATIENTS WITH ADVANCED HIV INFECTION AT RISK FOR CMV DISEASE, FOR MAINTENANCE TREATMENT OF CMV RETINITIS IN IMMUNOCOMPROMISED PATIENTS, AND FOR PREVENTION OF CMV DISEASE IN SOLID ORGAN TRANSPLANT RECIPIENTS (see INDICATIONS AND USAGE).
BECAUSE GANCICLOVIR CAPSULES ARE ASSOCIATED WITH A RISK OF MORE RAPID RATE OF CMV RETINITIS PROGRESSION, THEY SHOULD BE USED AS MAINTENANCE TREATMENT ONLY IN THOSE PATIENTS FOR WHOM THIS RISK IS BALANCED BY THE BENEFIT ASSOCIATED WITH AVOIDING DAILY INTRAVENOUS INFUSIONS.
-
DESCRIPTION
Ganciclovir is a synthetic guanine derivative active against cytomegalovirus (CMV).
Ganciclovir is available as 250 mg and 500 mg capsules. Each capsule contains 250 mg or 500 mg ganciclovir, USP respectively, and inactive ingredients croscarmellose sodium, FD&C blue #2, gelatin, iron oxide black, iron oxide yellow, lecithin, magnesium stearate, microcrystalline cellulose, povidone, shellac, simethicone, and titanium dioxide.
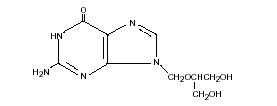
Ganciclovir is a white to off-white crystalline powder with a molecular formula of C9H13N504 and a molecular weight of 255.23. The chemical name for ganciclovir is 9-[[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl]guanine. Ganciclovir is a polar hydrophilic compound with a solubility of 2.6 mg/mL in water at 25° C and an n-octanol/water partition coefficient of 0.022. The pKas for ganciclovir are 2.2 and 9.4. The molecular structure of ganciclovir is:
All doses in this insert are specified in terms of ganciclovir.
-
VIROLOGY
Mechanism of Action: Ganciclovir is an acyclic nucleoside analogue of 2'-deoxyguanosine that inhibits replication of herpes viruses. Ganciclovir has been shown to be active against cytomegalovirus (CMV) and herpes simplex virus (HSV) in human clinical studies.
To achieve anti-CMV activity, ganciclovir is phosphorylated first to the monophosphate form by a CMV-encoded (UL97 gene) protein kinase homologue, then to the di- and triphosphate forms by cellular kinases. Ganciclovir triphosphate concentrations may be 100-fold greater in CMV-infected than in uninfected cells, indicating preferential phosphorylation in infected cells. Ganciclovir triphosphate, once formed, persists for days in the CMV-infected cell. Ganciclovir triphosphate is believed to inhibit viral DNA synthesis by (1) competitive inhibition of viral DNA polymerases; and (2) incorporation into viral DNA, resulting in eventual termination of viral DNA elongation.
Antiviral Activity: The median concentration of ganciclovir that inhibits CMV replication (IC50) in vitro (laboratory strains or clinical isolates) has ranged from 0.02 to 3.48 mcg/mL. Ganciclovir inhibits mammalian cell proliferation (CIC50) in vitro at higher concentrations ranging from 30 to 725 mcg/mL. Bone marrow-derived colony-forming cells are more sensitive (CIC50 0.028 to 0.7 mcg/mL). The relationship of in vitro sensitivity of CMV to ganciclovir and clinical response has not been established.
Clinical Antiviral Effect of Ganciclovir Capsules:In trials comparing ganciclovir IV with ganciclovir capsules for the maintenance treatment of CMV retinitis in patients with AIDS, serial urine cultures and other available cultures (semen, biopsy specimens, blood and others) showed that a small proportion of patients remained culture-positive during maintenance therapy with no statistically significant differences in CMV isolation rates between treatment groups.
A study of ganciclovir capsules (1000 mg q8h) for prevention of CMV disease in individuals with advanced HIV infection (ICM 1654) evaluated antiviral activity as measured by CMV isolation in culture; most cultures were from urine. At baseline, 40% (176/436) and 44% (92/210) of ganciclovir and placebo recipients, respectively, had positive cultures (urine or blood). After 2 months on treatment, 10% vs 44% of ganciclovir vs placebo recipients had positive cultures.
Viral Resistance: The current working definition of CMV resistance to ganciclovir in in vitro assays is IC50 > 3 mcg/mL (12 µM). CMV resistance to ganciclovir has been observed in individuals with AIDS and CMV retinitis who have never received ganciclovir therapy. In a controlled study of oral ganciclovir for prevention of AIDS-associated CMV disease, 364 individuals had one or more cultures performed after at least 90 days of ganciclovir treatment. Of these, 113 had at least one positive culture. The last available isolate from each subject was tested for reduced sensitivity, and 2 of 40 were found to be resistant to ganciclovir. These resistant isolates were associated with subsequent treatment failure for retinitis.
The possibility of viral resistance should be considered in patients who show poor clinical response or experience persistent viral excretion during therapy. The principal mechanism of resistance to ganciclovir in CMV is the decreased ability to form the active triphosphate moiety; resistant viruses have been described that contain mutations in the UL97 gene of CMV that controls phosphorylation of ganciclovir. Mutations in the viral DNA polymerase have also been reported to confer viral resistance to ganciclovir.
-
CLINICAL PHARMACOLOGY
Pharmacokinetics
BECAUSE THE MAJOR ELIMINATION PATHWAY FOR GANCICLOVIR IS RENAL, DOSAGE REDUCTIONS ACCORDING TO CREATININE CLEARANCE SHOULD BE CONSIDERED FOR GANCICLOVIR CAPSULES. FOR DOSING INSTRUCTIONS IN PATIENTS WITH RENAL IMPAIRMENT, REFER TO DOSAGE AND ADMINISTRATION.
Absorption: The absolute bioavailability of oral ganciclovir under fasting conditions was approximately 5% (n = 6) and following food was 6% to 9% (n = 32). When ganciclovir was administered orally with food at a total daily dosage of 3 g/day (500 mg q3h, 6 times daily and 1000 mg tid), the steady-state absorption as measured by area under the serum concentration vs time curve (AUC) over 24 hours and maximum serum concentrations (Cmax) were similar following both regimens with an AUC0-24 of 15.9 ± 4.2 (mean ± SD) and 15.4 ± 4.3 mcg•hr/mL and Cmax of 1.02 ± 0.24 and 1.18 ± 0.36 mcg/mL, respectively (n = 16).
Food Effects: When ganciclovir capsules were given with a meal containing 602 calories and 46.5% fat at a dosage of 1000 mg every 8 hours to 20 HIV-positive subjects, the steady-state AUC increased by 22 ± 22% (range: -6% to 68%) and there was a significant prolongation of time to peak serum concentrations (Tmax) from 1.8 ± 0.8 to 3 ± 0.6 hours and a higher Cmax (0.85 ± 0.25 vs 0.96 ± 0.27 mcg/mL) (n = 20).
Distribution: For ganciclovir capsules, no correlation was observed between AUC and reciprocal weight (range: 55 to 128 kg); oral dosing according to weight is not required. Binding to plasma proteins was 1% to 2% over ganciclovir concentrations of 0.5 and 51 mcg/mL.
Metabolism: Following oral administration of a single 1000 mg dose of 14C-labeled ganciclovir, 86 ± 3% of the administered dose was recovered in the feces and 5 ± 1% was recovered in the urine (n = 4). No metabolite accounted for more than 1% to 2% of the radioactivity recovered in urine or feces.
Elimination: When administered orally, it exhibits linear kinetics up to a total daily dose of 4 g/day. Renal excretion of unchanged drug by glomerular filtration and active tubular secretion is the major route of elimination of ganciclovir. After oral administration of ganciclovir, steady-state is achieved within 24 hours. Renal clearance following oral administration was 3.1 ± 1.2 mL/min/kg (n = 22). Half-life was 4.8 ± 0.9 hours (n = 39) following oral administration.
Special Populations: The pharmacokinetics of ganciclovir following oral administration of ganciclovir capsules were evaluated in 44 patients, who were either solid organ transplant recipients or HIV positive. Apparent oral clearance of ganciclovir decreased and AUC0-24h increased with diminishing renal function (as expressed by creatinine clearance).
Based on these observations, it is necessary to modify the dosage of ganciclovir in patients with renal impairment (see DOSAGE AND ADMINISTRATION).
Hemodialysis reduces plasma concentrations of ganciclovir by about 50% after oral administration.
Race/Ethnicity and Gender: The effects of race/ethnicity and gender were studied in subjects receiving a dose regimen of 1000 mg every 8 hours. Although the numbers of blacks (16%) and Hispanics (20%) were small, there appeared to be a trend towards a lower steady-state Cmax and AUC0-8 in these subpopulations as compared to Caucasians. No definitive conclusions regarding gender differences could be made because of the small number of females (12%); however, no differences between males and females were observed.
Elderly: No studies have been conducted in adults older than 65 years of age.
-
INDICATIONS AND USAGE
Ganciclovir capsules are indicated for the prevention of CMV disease in solid organ transplant recipients and in individuals with advanced HIV infection at risk for developing CMV disease. Ganciclovir capsules are also indicated as an alternative to the intravenous formulation for maintenance treatment of CMV retinitis in immunocompromised patients, including patients with AIDS, in whom retinitis is stable following appropriate induction therapy and for whom the risk of more rapid progression is balanced by the benefit associated with avoiding daily IV infusions (see CLINICAL TRIALS).
SAFETY AND EFFICACY OF GANCICLOVIR HAVE NOT BEEN ESTABLISHED FOR CONGENITAL OR NEONATAL CMV DISEASE; NOT FOR THE TREATMENT OF ESTABLISHED CMV DISEASE OTHER THAN RETINITIS; NOR FOR USE IN NON-IMMUNOCOMPROMISED INDIVIDUALS. THE SAFETY AND EFFICACY OF GANCICLOVIR CAPSULES HAVE NOT BEEN ESTABLISHED FOR TREATING ANY MANIFESTATION OF CMV DISEASE OTHER THAN MAINTENANCE TREATMENT OF CMV RETINITIS.
-
CLINICAL TRIALS
The diagnosis of CMV retinitis should be made by indirect ophthalmoscopy. Other conditions in the differential diagnosis of CMV retinitis include candidiasis, toxoplasmosis, histoplasmosis, retinal scars and cotton wool spots, any of which may produce a retinal appearance similar to CMV. For this reason it is essential that the diagnosis of CMV be established by an ophthalmologist familiar with the retinal presentation of these conditions. The diagnosis of CMV retinitis may be supported by culture of CMV from urine, blood, throat or other sites, but a negative CMV culture does not rule out CMV retinitis.
Studies Comparing Ganciclovir-Capsules to Ganciclovir-IV:
Population Characteristics in Studies ICM 1653, ICM 1774 and AVI 034
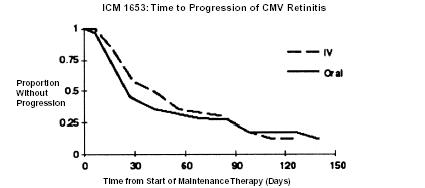
ICM 1653 (n = 121) ICM 1774 (n = 225) AVI 034 (n = 159) Median age (years) Range 38 37 39 24 to 62 22 to 56 23 to 62 Sex Males 116 (96%) 222 (99%) 148 (93%) Female 5 (4%) 3 (1%) 10 (6%) Ethnicity Asian 3 (3%) 5 (2%) 7 (4%) Black 11 (9%) 9 (4%) 3 (2%) Caucasian 98 (81%) 186 (83%) 140 (88%) Other 9 (7%) 25 (11%) 8 (5%) Median CD4 Count Range 9.5 7 10 0 to 141 0 to 80 0 to 320 Mean (SD) Observation Time (days) 107.9 (43) 97.6 (42.5) 80.9 (47) ICM 1653: In this randomized, open-label, parallel group trial, conducted between March 1991 and November 1992, patients with AIDS and newly diagnosed CMV retinitis received a 3-week induction course of ganciclovir- IV solution, 5 mg/kg bid for 14 days followed by 5 mg/kg once daily for 1 additional week.1 Following the 21-day intravenous induction course, patients with stable CMV retinitis were randomized to receive 20 weeks of maintenance treatment with either ganciclovir-IV solution, 5 mg/kg once daily, or ganciclovir capsules, 500 mg 6 times daily (3000 mg/day). The study showed that the mean [95% CI] and median [95% CI] times to progression of CMV retinitis, as assessed by masked reading of fundus photographs, were 57 days [44, 70] and 29 days [28, 43], respectively, for patients on oral therapy compared to 62 days [50, 73] and 49 days [29, 61], respectively, for patients on intravenous therapy. The difference [95% CI] in the mean time to progression between the oral and intravenous therapies (oral - IV) was -5 days [-22, 12]. See Figure 1 for comparison of the proportion of patients remaining free of progression over time.
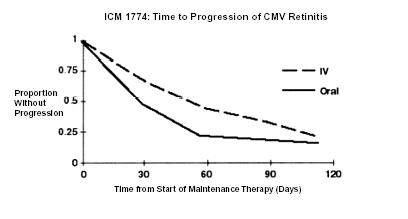
ICM 1774: In this three-arm, randomized, open-label, parallel group trial, conducted between June 1991 and August 1993, patients with AIDS and stable CMV retinitis following from 4 weeks to 4 months of treatment with ganciclovir-IV solution were randomized to receive maintenance treatment with ganciclovir-IV solution, 5 mg/kg once daily, ganciclovir capsules, 500 mg 6 times daily, or ganciclovir capsules, 1000 mg tid for 20 weeks. The study showed that the mean [95% CI] and median [95% CI] times to progression of CMV retinitis, as assessed by masked reading of fundus photographs, were 54 days [48, 60] and 42 days [31, 54], respectively, for patients on oral therapy compared to 66 days [56, 76] and 54 days [41, 69], respectively, for patients on intravenous therapy. The difference [95% CI] in the mean time to progression between the oral and intravenous therapies (oral - IV) was -12 days [-24, 0]. See Figure 2 for comparison of the proportion of patients remaining free of progression over time.
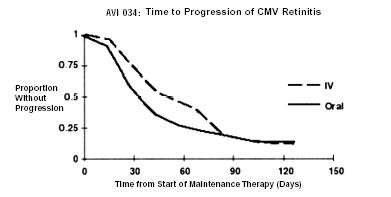
AVI 034: In this randomized, open-label, parallel group trial, conducted between June 1991 and February 1993, patients with AIDS and newly diagnosed (81%) or previously treated (19%) CMV retinitis who had tolerated 10 to 21 days of induction treatment with ganciclovir-IV, 5 mg/kg twice daily, were randomized to receive 20 weeks of maintenance treatment with either ganciclovir capsules, 500 mg 6 times daily or ganciclovir-IV solution, 5 mg/kg/day.2 The mean [95% CI] and median [95% CI] times to progression of CMV retinitis, as assessed by masked reading of fundus photographs, were 51 days [44, 57] and 41 days [31, 45], respectively, for patients on oral therapy compared to 62 days [52, 72] and 60 days [42, 83], respectively, for patients on intravenous therapy. The difference [95% CI] in the mean time to progression between the oral and intravenous therapies (oral - IV) was -11 days [-24, 1]. See Figure 3 for comparison of the proportion of patients remaining free of progression over time.
Comparison of other CMV retinitis outcomes between oral and IV formulations (development of bilateral retinitis, progression into Zone 1, and deterioration of visual acuity), while not definitive, showed no marked differences between treatment groups in these studies. Because of low event rates among these endpoints, these studies are underpowered to rule out significant differences in these endpoints.
2. Prevention of CMV Disease in Subjects With AIDS
ICM 1654: In a double-blind study conducted between November 1992 and July 1994, 725 subjects with AIDS, who were CMV seropositive and/or culture positive, were randomized to receive ganciclovir capsules, 1000 mg, every 8 hours, or placebo.3 The study population had a median age of 38 years (range: 21 to 69); were 99% male; were 82% Caucasian, 10% Hispanic, 7% African-American and 1% Asian; and had a median CD4 count of 21 (range: 0 to 100). The mean observation time was 351 days (range: 5 to 621). As shown in the following table, significantly more placebo recipients developed CMV disease.
Incidence of CMV Disease at 6, 12 and 18 Months After Enrollment (Kaplan-Meier Estimates)
Incidence (Number Still at Risk) CMV Disease Ganciclovir Placebo 6 months 8% (397) 11% (190) 12 months 14% (225) 26% (92) 18 months 20% (27) 39% (9) 3. Prevention of CMV Disease in Transplant Recipients
GAN040: Ganciclovir capsules were evaluated in a randomized, double-blind, placebo-controlled study of 304 orthotopic liver transplant recipients who were CMV seropositive or recipients of an organ from a seropositive donor. Administration of ganciclovir capsules (1000 mg three times daily) or matching placebo commenced as soon as patients were able to take medication by mouth, but no later than 10 days following transplantation, and continued through 14 weeks after transplantation. Dosing was adjusted for patients with an estimated creatinine clearance < 50 mL/min. The incidence of CMV disease at 6 months is summarized in the table below:
Incidence of CMV Disease at 6 Months (Kaplan-Meier Estimates)
CMV Disease at 6 months Ganciclovir (n = 150) Placebo (n = 154) Relative Risk (95% Cl) CMV Disease,* N (%) 7 (4.8%) 29 (18.9%) 0.22 (0.10, 0.51) CMV syndrome# 6 (4.1%) 19 (12.4%) CMV hepatitis 1 (0.7%) 9 (5.9%) CMV GI disease 0 (0%) 3 (2%) CMV lung disease 0 (0%) 4 (2.6%) # CMV syndrome: CMV viremia and unexplained fever, accompanied by malaise and/or neutropenia.
Ganciclovir capsules significantly reduced the 6-month incidence of CMV disease in patients at increased risk of CMV disease, including seronegative recipients of organs from seropositive donors (15% [3/21] with ganciclovir capsules vs 44% [11/25] with placebo), and patients receiving antilymphocyte antibodies (5% [2/44] with ganciclovir capsules vs 33% [12/37] with placebo). The incidence of HSV infection at 6 months was 4% (5/150) in ganciclovir vs 24% (36/154) in placebo recipients (relative risk: 0.13; 95% CI: 0.05, 0.32).
- CONTRAINDICATIONS
-
WARNINGS
Hematologic: Ganciclovir should not be administered if the absolute neutrophil count is less than 500 cells/µL or the platelet count is less than 25,000 cells/µL. Granulocytopenia (neutropenia), anemia and thrombocytopenia have been observed in patients treated with ganciclovir. The frequency and severity of these events vary widely in different patient populations (see ADVERSE EVENTS).
Ganciclovir should, therefore, be used with caution in patients with pre-existing cytopenias or with a history of cytopenic reactions to other drugs, chemicals or irradiation. Granulocytopenia usually occurs during the first or second week of treatment but may occur at any time during treatment. Cell counts usually begin to recover within 3 to 7 days of discontinuing drug.
Impairment of Fertility: Animal data indicate that administration of ganciclovir causes inhibition of spermatogenesis and subsequent infertility. These effects were reversible at lower doses and irreversible at higher doses (see PRECAUTIONS: Carcinogenesis, Mutagenesis and Impairment of Fertility). Although data in humans have not been obtained regarding this effect, it is considered probable that ganciclovir at the recommended doses causes temporary or permanent inhibition of spermatogenesis. Animal data also indicate that suppression of fertility in females may occur.
Teratogenesis: Because of the mutagenic and teratogenic potential of ganciclovir, women of childbearing potential should be advised to use effective contraception during treatment. Similarly, men should be advised to practice barrier contraception during and for at least 90 days following treatment with ganciclovir (see Pregnancy; Teratogenic Effects: Category C).
-
PRECAUTIONS
General
Since ganciclovir is excreted by the kidneys, normal clearance depends on adequate renal function. IF RENAL FUNCTION IS IMPAIRED, DOSAGE ADJUSTMENTS SHOULD BE CONSIDERED FOR GANCICLOVIR CAPSULES. Such adjustments should be based on measured or estimated creatinine clearance values (see DOSAGE AND ADMINISTRATION).
Information for Patients
All patients should be informed that the major toxicities of ganciclovir are granulocytopenia (neutropenia), anemia and thrombocytopenia and that dose modifications may be required, including discontinuation. The importance of close monitoring of blood counts while on therapy should be emphasized. Patients should be informed that ganciclovir has been associated with elevations in serum creatinine.
Patients should be instructed to take ganciclovir capsules with food to maximize bioavailability.
Patients should be advised that ganciclovir has caused decreased sperm production in animals and may cause infertility in humans. Women of childbearing potential should be advised that ganciclovir causes birth defects in animals and should not be used during pregnancy. Women of childbearing potential should be advised to use effective contraception during treatment with ganciclovir. Similarly, men should be advised to practice barrier contraception during and for at least 90 days following treatment with ganciclovir.
Patients should be advised that ganciclovir causes tumors in animals. Although there is no information from human studies, ganciclovir should be considered a potential carcinogen.
All HIV+ Patients: These patients may be receiving zidovudine (Retrovir®*). Patients should be counseled that treatment with both ganciclovir and zidovudine simultaneously may not be tolerated by some patients and may result in severe granulocytopenia (neutropenia). Patients with AIDS may be receiving didanosine (Videx®#). Patients should be counseled that concomitant treatment with both ganciclovir and didanosine can cause didanosine serum concentrations to be significantly increased.
HIV+ Patients With CMV Retinitis: Ganciclovir is not a cure for CMV retinitis, and immunocompromised patients may continue to experience progression of retinitis during or following treatment. Patients should be advised to have ophthalmologic follow-up examinations at a minimum of every 4 to 6 weeks while being treated with ganciclovir. Some patients will require more frequent follow-up.
Laboratory Testing
Due to the frequency of neutropenia, anemia and thrombocytopenia in patients receiving ganciclovir (see ADVERSE EVENTS), it is recommended that complete blood counts and platelet counts be performed frequently, especially in patients in whom ganciclovir or other nucleoside analogues have previously resulted in leukopenia, or in whom neutrophil counts are less than 1000 cells/µL at the beginning of treatment. Increased serum creatinine levels have been observed in trials evaluating ganciclovir. Patients should have serum creatinine or creatinine clearance values monitored carefully to allow for dosage adjustments in renally impaired patients (see DOSAGE AND ADMINISTRATION).
Drug Interactions
Didanosine: At an oral dose of 1000 mg of ganciclovir every 8 hours and didanosine, 200 mg every 12 hours, the steady-state didanosine AUC0-12 increased 111 ± 114% (range: 10% to 493%) when didanosine was administered either 2 hours prior to or concurrent with administration of ganciclovir (n = 12 patients, 23 observations). A decrease in steady-state ganciclovir AUC of 21 ± 17% (range: -44% to 5%) was observed when didanosine was administered 2 hours prior to administration of ganciclovir, but ganciclovir AUC was not affected by the presence of didanosine when the two drugs were administered simultaneously (n = 12). There were no significant changes in renal clearance for either drug.
Zidovudine: At an oral dose of 1000 mg of ganciclovir every 8 hours, mean steady-state ganciclovir AUC0-8 decreased 17 ± 25% (range: -52% to 23%) in the presence of zidovudine, 100 mg every 4 hours (n = 12). Steady-state zidovudine AUC0-4 increased 19 ± 27% (range: -11% to 74%) in the presence of ganciclovir.
Since both zidovudine and ganciclovir have the potential to cause neutropenia and anemia, some patients may not tolerate concomitant therapy with these drugs at full dosage.
Probenecid: At an oral dose of 1000 mg of ganciclovir every 8 hours (n = 10), ganciclovir AUC0-8 increased 53 ± 91% (range: -14% to 299%) in the presence of probenecid, 500 mg every 6 hours. Renal clearance of ganciclovir decreased 22 ± 20% (range: -54% to -4%), which is consistent with an interaction involving competition for renal tubular secretion.
Imipenem-cilastatin: Generalized seizures have been reported in patients who received ganciclovir and imipenem-cilastatin. These drugs should not be used concomitantly unless the potential benefits outweigh the risks.
Other Medications: It is possible that drugs that inhibit replication of rapidly dividing cell populations such as bone marrow, spermatogonia and germinal layers of skin and gastrointestinal mucosa may have additive toxicity when administered concomitantly with ganciclovir. Therefore, drugs such as dapsone, pentamidine, flucytosine, vincristine, vinblastine, adriamycin, amphotericin B, trimethoprim/sulfamethoxazole combinations or other nucleoside analogues, should be considered for concomitant use with ganciclovir only if the potential benefits are judged to outweigh the risks.
No formal drug interaction studies of ganciclovir and drugs commonly used in transplant recipients have been conducted.
Carcinogenesis, MutagenesisT
Ganciclovir was carcinogenic in the mouse at oral doses of 20 and 1000 mg/kg/day (approximately 0.1x and 1.4x, respectively, the mean drug exposure in humans following the recommended intravenous dose of 5 mg/kg, based on area under the plasma concentration curve [AUC] comparisons). At the dose of 1000 mg/kg/day there was a significant increase in the incidence of tumors of the preputial gland in males, forestomach (nonglandular mucosa) in males and females, and reproductive tissues (ovaries, uterus, mammary gland, clitoral gland and vagina) and liver in females. At the dose of 20 mg/kg/day, a slightly increased incidence of tumors was noted in the preputial and harderian glands in males, forestomach in males and females, and liver in females. No carcinogenic effect was observed in mice administered ganciclovir at 1 mg/kg/day (estimated as 0.01x the human dose based on AUC comparison). Except for histiocytic sarcoma of the liver, ganciclovir- induced tumors were generally of epithelial or vascular origin. Although the preputial and clitoral glands, forestomach and harderian glands of mice do not have human counterparts, ganciclovir should be considered a potential carcinogen in humans.
Ganciclovir increased mutations in mouse lymphoma cells and DNA damage in human lymphocytes in vitro at concentrations between 50 to 500 and 250 to 2000 mcg/mL, respectively. Ganciclovir was not mutagenic in the Ames Salmonella assay at concentrations of 500 to 5000 mcg/mL.
Impairment of FertilityT
Ganciclovir caused decreased fertility in male mice and hypospermatogenesis in mice and dogs following daily administration of doses ranging from 0.2 to 10 mg/kg.
Pregnancy: Teratogenic Effects: Pregnancy Category CT
Ganciclovir has been shown to be embryotoxic in rabbits, mice and teratogenic in rabbits. Fetal resorptions were present in at least 85% of rabbits and mice administered 60 mg/kg/day and 108 mg/kg/day (2 x the human exposure based on AUC comparisons), respectively.
Effects observed in rabbits included: fetal growth retardation, embryolethality, teratogenicity and/or maternal toxicity. Teratogenic changes included cleft palate, anophthalmia/microphthalmia, aplastic organs (kidney and pancreas), hydrocephaly and brachygnathia. In mice, effects observed were maternal/fetal toxicity and embryolethality.
Ganciclovir may be teratogenic or embryotoxic at dose levels recommended for human use. There are no adequate and well-controlled studies in pregnant women. Ganciclovir should be used during pregnancy only if the potential benefits justify the potential risk to the fetus.
TFootnote: Compared with the single 5 mg/kg intravenous infusion, human exposure is doubled during the intravenous induction phase (5 mg/kg bid) and approximately halved during maintenance treatment with ganciclovir capsules (1000 mg tid). The cross-species dosage treatment should be multiplied by 2 for ganciclovir capsules.
Nursing Mothers
It is not known whether ganciclovir is excreted in human milk. However, many drugs are excreted in human milk and, because carcinogenic and teratogenic effects occurred in animals treated with ganciclovir, the possibility of serious adverse reactions from ganciclovir in nursing infants is considered likely (see Pregnancy: Teratogenic Effects: Pregnancy Category C). Mothers should be instructed to discontinue nursing if they are receiving ganciclovir. The minimum interval before nursing can safely be resumed after the last dose of ganciclovir is unknown.
Pediatric Use
SAFETY AND EFFICACY OF GANCICLOVIR IN PEDIATRIC PATIENTS HAVE NOT BEEN ESTABLISHED. THE USE OF GANCICLOVIR IN THE PEDIATRIC POPULATION WARRANTS EXTREME CAUTION DUE TO THE PROBABILITY OF LONG-TERM CARCINOGENICITY AND REPRODUCTIVE TOXICITY. ADMINISTRATION TO PEDIATRIC PATIENTS SHOULD BE UNDERTAKEN ONLY AFTER CAREFUL EVALUATION AND ONLY IF THE POTENTIAL BENEFITS OF TREATMENT OUTWEIGH THE RISKS.
Ganciclovir capsules have not been studied in pediatric patients under age 13.
Geriatric Use
The pharmacokinetic profiles of ganciclovir in elderly patients have not been established. Since elderly individuals frequently have a reduced glomerular filtration rate, particular attention should be paid to assessing renal function before and during administration of ganciclovir (see DOSAGE AND ADMINISTRATION).
Clinical studies of ganciclovir did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Ganciclovir is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection. In addition, renal function should be monitored and dosage adjustments should be made accordingly (see Use in Patients With Renal Impairmentand DOSAGE AND ADMINISTRATION)
Use in Patients With Renal Impairment
Ganciclovir should be used with caution in patients with impaired renal function because the half-life and plasma/serum concentrations of ganciclovir will be increased due to reduced renal clearance (see DOSAGE AND ADMINISTRATION and ADVERSE EVENTS: Renal Toxicity).
Hemodialysis has been shown to reduce plasma levels of ganciclovir by approximately 50%.
-
ADVERSE REACTIONS
Adverse events that occurred during clinical trials of ganciclovir capsules are summarized below, according to the participating study subject population.
Subjects With AIDS
Three controlled, randomized, phase 3 trials comparing ganciclovir-IV and ganciclovir capsules for maintenance treatment of CMV retinitis have been completed. During these trials, ganciclovir-IV or ganciclovir capsules were prematurely discontinued in 9% of subjects because of adverse events. In a placebo-controlled, randomized, phase 3 trial of ganciclovir capsules for prevention of CMV disease in AIDS, treatment was prematurely discontinued because of adverse events, new or worsening intercurrent illness, or laboratory abnormalities in 19.5% of subjects treated with ganciclovir capsules and 16% of subjects receiving placebo. Laboratory data and adverse events reported during the conduct of these controlled trials are summarized below.
Selected Laboratory Abnormalities in Trials for Treatment of CMV Retinitis and Prevention of CMV Disease
Adverse events: The following table shows selected adverse events reported in 5% or more of the subjects in three controlled clinical trials during treatment with ganciclovir capsules (3000 mg/day) and in one controlled clinical trial in which ganciclovir capsules (3000 mg/day) were compared to placebo for the prevention of CMV disease.
Selected Adverse Events Reported in ≥ 5% of Subjects in Three Randomized Phase 3 Studies Comparing Ganciclovir Capsules to Ganciclovir-IV Solution for Maintenance Treatment of CMV Retinitis and in One Phase 3 Randomized Study Comparing Ganciclovir Capsules to Placebo for Prevention of CMV Disease Maintenance Treatment Studies Prevention Study Body System Adverse Event Capsules (n = 326) IV (n = 179) Capsules (n = 478) Placebo (n = 234) Body as a Whole Fever 38% 48% 35% 33% Infection 9% 13% 8% 4% Chills 7% 10% 7% 4% Sepsis 4% 15% 3% 2% Digestive System Diarrhea 41% 44% 48% 42% Anorexia 15% 14% 19% 16% Vomiting 13% 13% 14% 11% Hemic and Lymphatic System Leukopenia 29% 41% 17% 9% Anemia 19% 25% 9% 7% Thrombocytopenia 6% 6% 3% 1% Nervous System Neuropathy 8% 9% 21% 15% Other Sweating 11% 12% 14% 12% Pruritus 6% 6% 10% 9% Catheter Related* Total Catheter Events 6% 22% – – Catheter Infection 4% 9% – – Catheter Sepsis 1% 8% – – The following events were frequently observed in clinical trials but occurred with equal or greater frequency in placebo-treated subjects: abdominal pain, nausea, flatulence, pneumonia, paresthesia, rash.
Retinal Detachment: Retinal detachment has been observed in subjects with CMV retinitis both before and after initiation of therapy with ganciclovir. Its relationship to therapy with ganciclovir is unknown. Retinal detachment occurred in 8% of patients treated with ganciclovir capsules. Patients with CMV retinitis should have frequent ophthalmologic evaluations to monitor the status of their retinitis and to detect any other retinal pathology.
Transplant Recipients
There has been one controlled clinical trial of ganciclovir capsules for the prevention of CMV disease in transplant recipients. Laboratory data and adverse events reported during these trials are summarized below.
Laboratory Data: The following table shows the frequency of granulocytopenia (neutropenia) and thrombocytopenia observed:
The following table shows the frequency of elevated serum creatinine values in these controlled clinical trials:
Controlled Trials – Transplant Recipients Ganciclovir Capsules Liver Allograft Study 040 Maximum Serum Creatinine Levels Ganciclovir Capsules (n = 150) Placebo (n = 154) Serum Creatinine ≥ 2.5 mg/dL 16% 10% Serum Creatinine ≥ 1.5 to < 2.5 mg/dL 39% 42% In 3 out of 4 trials, patients receiving either ganciclovir-IV solution or ganciclovir capsules had elevated serum creatinine levels when compared to those receiving placebo. Most patients in these studies also received cyclosporine. The mechanism of impairment of renal function is not known. However, careful monitoring of renal function during therapy with ganciclovir capsules is essential, especially for those patients receiving concomitant agents that may cause nephrotoxicity.
General
Other adverse events that were thought to be “probably” or “possibly” related to ganciclovir capsules in controlled clinical studies in either subjects with AIDS or transplant recipients are listed below. These events all occurred in at least 3 subjects.
Body as a Whole: abdomen enlarged, asthenia, chest pain, edema, headache, injection site inflammation, malaise, pain
Digestive System: abnormal liver function test, aphthous stomatitis, constipation, dyspepsia, eructation
Hemic and Lymphatic System: pancytopenia
Respiratory System: cough increased, dyspnea
Nervous System: abnormal dreams, anxiety, confusion, depression, dizziness, dry mouth, insomnia, seizures, somnolence, thinking abnormal, tremor
Skin and Appendages: alopecia, dry skin
Special Senses: abnormal vision, taste perversion, tinnitus, vitreous disorder
Metabolic and Nutritional Disorders: creatinine increased, SGOT increased, SGPT increased, weight loss
Cardiovascular System: hypertension, phlebitis, vasodilatation
Urogenital System: creatinine clearance decreased, kidney failure, kidney function abnormal, urinary frequency
Musculoskeletal System: arthralgia, leg cramps, myalgia, myasthenia
The following adverse events reported in patients receiving ganciclovir may be potentially fatal: gastrointestinal perforation, multiple organ failure, pancreatitis and sepsis.
Adverse Events Reported During Postmarketing Experience With Ganciclovir Capsules
The following events have been identified during postapproval use of the drug. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to either the seriousness, frequency of reporting, the apparent causal connection or a combination of these factors:
acidosis, allergic reaction, anaphylactic reaction, arthritis, bronchospasm, cardiac arrest, cardiac conduction abnormality, cataracts, cholelithiasis, cholestasis, congenital anomaly, dry eyes, dysesthesia, dysphasia, elevated triglyceride levels, encephalopathy, exfoliative dermatitis, extrapyramidal reaction, facial palsy, hallucinations, hemolytic anemia, hemolytic uremic syndrome, hepatic failure, hepatitis, hypercalcemia, hyponatremia, inappropriate serum ADH, infertility, intestinal ulceration, intracranial hypertension, irritability, loss of memory, loss of sense of smell, myelopathy, oculomotor nerve paralysis, peripheral ischemia, pulmonary fibrosis, renal tubular disorder, rhabdomyolysis, Stevens-Johnson syndrome, stroke, testicular hypotrophy, Torsades de Pointes, vasculitis, ventricular tachycardia
-
OVERDOSAGE
There have been no reports of overdosage with ganciclovir capsules. Doses as high as 6000 mg/day, given either as 1000 mg 6 times daily or as 2000 mg tid, did not result in overt toxicity other than transient neutropenia. Daily doses of more than 6000 mg have not been studied.
Since ganciclovir is dialyzable, dialysis may be useful in reducing serum concentrations. Adequate hydration should be maintained. The use of hematopoietic growth factors should be considered.
-
DOSAGE AND ADMINISTRATION
Dosage: THE RECOMMENDED DOSE FOR GANCICLOVIR CAPSULES SHOULD NOT BE EXCEEDED.
For Treatment of CMV Retinitis in Patients WithNormal Renal Function:
Ganciclovir capsules should not be used for induction treatment.
Following induction treatment, the recommended maintenance dosage of ganciclovir capsules is 1000 mg tid with food. Alternatively, the dosing regimen of 500 mg 6 times daily every 3 hours with food, during waking hours, may be used.
For patients who experience progression of CMV retinitis while receiving maintenance treatment with either formulation of ganciclovir, reinduction treatment is recommended.
For the Prevention of CMV Disease in Patients With Advanced HIV Infection andNormalRenal Function:
The recommended prophylactic dose of ganciclovir capsules is 1000 mg tid with food.
For the Prevention of CMV Disease in Transplant Recipients With Normal Renal Function:
The recommended prophylactic dosage of ganciclovir capsules is 1000 mg tid with food.
The duration of treatment with ganciclovir capsules in transplant recipients is dependent upon the duration and degree of immunosuppression. In a controlled clinical trial of liver allograft recipients, treatment with ganciclovir capsules was continued through week 14 posttransplantation (see INDICATIONS AND USAGE section for a more detailed discussion).
In patients with renal impairment, the dose of ganciclovir capsules should be modified as shown below:
Creatinine clearance for males = (140 - age [yrs]) (body wt [kg]) (72) (serum creatinine [mg/dL] Creatinine clearance for females = 0.85 x male value
Patient Monitoring: Due to the frequency of granulocytopenia, anemia and thrombocytopenia in patients receiving ganciclovir (see ADVERSE EVENTS), it is recommended that complete blood counts and platelet counts be performed frequently, especially in patients in whom ganciclovir or other nucleoside analogues have previously resulted in cytopenia, or in whom neutrophil counts are less than 1000 cells/µL at the beginning of treatment. Patients should have serum creatinine or creatinine clearance values followed carefully to allow for dosage adjustments in renally impaired patients (see DOSAGE AND ADMINISTRATION).
Reduction of Dose: Dosage reductions in renally impaired patients should be considered for ganciclovir capsules (see Renal Impairment). Dosage reductions should also be considered for those with neutropenia, anemia and/or thrombocytopenia (see ADVERSE EVENTS). Ganciclovir should not be administered in patients with severe neutropenia (ANC less than 500/µL) or severe thrombocytopenia (platelets less than 25,000/µL).
Handling and Disposal: Caution should be exercised in the handling of ganciclovir capsules. Avoid direct contact with the skin or mucous membranes of the powder contained in ganciclovir capsules. If such contact occurs, wash thoroughly with soap and water; rinse eyes thoroughly with plain water. Ganciclovir capsules should not be opened or crushed.
Because ganciclovir shares some of the properties of antitumor agents (ie, carcinogenicity and mutagenicity), consideration should be given to handling and disposal according to guidelines issued for antineoplastic drugs. Several guidelines on this subject have been published.
There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
-
HOW SUPPLIED
Ganciclovir 250 mg capsules are opaque green cap/opaque green body; size ‘1’ hard gelatin capsules, printed “RX636” on cap and body in black ink, containing white to off-white granular powder. They are supplied as follows:
NDC 63304-636-60 Bottles of 60
NDC 63304-636-90 Bottles of 90
NDC 63304-636-28 Bottles of 180
NDC 63304-636-10 Bottles of 1000
Ganciclovir 500 mg capsules are opaque ivory yellow cap/opaque green body, size ‘0’ elongated hard gelatin capsules, printed “RX637” on cap and body in black ink, containing white to off-white granular powder. They are supplied as follows:
NDC 63304-637-60 Bottles of 60
NDC 63304-637-90 Bottles of 90
NDC 63304-637-28 Bottles of 180
NDC 63304-637-05 Bottles of 500
Store at 20 - 25° C (68 - 77° F) [See USP Controlled Room Temperature].
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
-
REFERENCES
1. Drew WL, Ives D, Lalezari JP, et al. Oral ganciclovir as maintenance treatment for cytomegalovirus retinitis in patients with AIDS. New Engl J Med. 1995; 333:615-620.
2. The Oral Ganciclovir European and Australian Cooperative Study Group. Intravenous vs oral ganciclovir: European/Australian comparative study of efficacy and safety in the prevention of cytomegalovirus retinitis recurrence in patients with AIDS. AIDS. 1995; 9:471-477.
3. Spector SA, McKinley GF, Lalezari JP, Samo T, et al. Oral ganciclovir for the prevention of cytomegalovirus disease in persons with AIDS. New Engl J Med. 1996; 334:1491-1497.
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GANCICLOVIR
ganciclovir capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63304-636 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GANCICLOVIR (UNII: P9G3CKZ4P5) (GANCICLOVIR - UNII:P9G3CKZ4P5) GANCICLOVIR 250 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) GELATIN (UNII: 2G86QN327L) FERRIC OXIDE BLACK (UNII: XM0M87F357) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) SHELLAC (UNII: 46N107B71O) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color green (opaque green cap & body) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code RX636 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63304-636-60 60 in 1 BOTTLE 2 NDC:63304-636-90 90 in 1 BOTTLE 3 NDC:63304-636-28 180 in 1 BOTTLE 4 NDC:63304-636-10 1000 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076457 08/27/2003 GANCICLOVIR
ganciclovir capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63304-637 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GANCICLOVIR (UNII: P9G3CKZ4P5) (GANCICLOVIR - UNII:P9G3CKZ4P5) GANCICLOVIR 500 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) GELATIN (UNII: 2G86QN327L) FERRIC OXIDE BLACK (UNII: XM0M87F357) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) SHELLAC (UNII: 46N107B71O) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color green (body) , yellow (cap) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code RX637 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63304-637-60 60 in 1 BOTTLE 2 NDC:63304-637-90 90 in 1 BOTTLE 3 NDC:63304-637-28 180 in 1 BOTTLE 4 NDC:63304-637-05 500 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076457 08/27/2003 Labeler - Ranbaxy Pharmaceuticals Inc. (937890044) Registrant - Ranbaxy Pharmaceuticals Inc. (937890044) Establishment Name Address ID/FEI Business Operations Ranbaxy Laboratories Limited 650456754 manufacture