Label: THYROGEN- thyrotropin alfa injection, powder, lyophilized, for solution
THYROGEN- thyrotropin alfa kit
- NDC Code(s): 58468-0030-1, 58468-0030-2, 58468-1849-4
- Packager: Genzyme Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated February 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use THYROGEN safely and effectively. See full prescribing information for THYROGEN.
THYROGEN® (thyrotropin alfa) for injection, for intramuscular use
Initial U.S. Approval: 1998INDICATIONS AND USAGE
THYROGEN® is a thyroid stimulating hormone indicated for:
-
Adjunctive Diagnostic Tool for Well-Differentiated Thyroid Cancer:
Use as an adjunctive diagnostic tool for serum thyroglobulin (Tg) testing with or without radioiodine imaging in the follow-up of patients with well-differentiated thyroid cancer who have previously undergone thyroidectomy. (1.1)
Limitations of Use:- THYROGEN-stimulated Tg levels are generally lower than, and do not correlate with Tg levels after thyroid hormone withdrawal.
- Even when THYROGEN-Tg testing is performed in combination with radioiodine imaging, there remains a risk of missing a diagnosis of thyroid cancer or underestimating the extent of the disease.
- Anti-Tg Antibodies may confound the Tg assay and render Tg levels uninterpretable.
-
Adjunct for Thyroid Remnant Ablation in Well-Differentiated Thyroid Cancer: Use as an adjunctive treatment for radioiodine ablation of thyroid tissue remnants in patients who have undergone a near-total or total thyroidectomy for well-differentiated thyroid cancer and who do not have evidence of distant metastatic thyroid cancer. (1.2)
Limitations of Use:- The effect of THYROGEN on thyroid cancer recurrence greater than 5 years post-remnant ablation has not been evaluated.
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
For injection: 0.9 mg of thyrotropin alfa as a lyophilized powder in a single-dose vial. (3)
CONTRAINDICATIONS
- If THYROGEN is administered with radioiodine, the contraindications to radioiodine also apply to this combination regimen. (4)
WARNINGS AND PRECAUTIONS
- THYROGEN-induced hyperthyroidism: Hospitalization for administration of THYROGEN and postadministrative observation should be considered for patients at risk. (5.1)
- Stroke: Stroke in female patients as well as other neurologic events in patients with central nervous system metastases. (5.2, 5.3)
- Sudden rapid tumor enlargement: Sudden, rapid and painful enlargement in distant metastatic thyroid cancer. (5.3)
- Risks associated with radioiodine (RAI) combination treatment: If THYROGEN is administered with RAI, the warnings and precautions for RAI also apply to this combination regimen. (5.4)
ADVERSE REACTIONS
The most common adverse reactions (>5%) reported in clinical trials were nausea and headache. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Genzyme Corporation at 800-745-4447 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Pregnancy: Concomitant use of THYROGEN and radioiodine (RAI) is contraindicated in pregnancy. (4, 8.1)
- Lactation: Concomitant use of THYROGEN and therapeutic RAI is contraindicated in lactating women. (4, 8.2)
- Renal Impairment: Elimination of THYROGEN is significantly slower in dialysis-dependent end-stage renal disease patients, resulting in prolonged elevation of TSH levels. (8.6)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2023
-
Adjunctive Diagnostic Tool for Well-Differentiated Thyroid Cancer:
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Adjunctive Diagnostic Tool for Well-Differentiated Thyroid Cancer
1.2 Adjunct for Thyroid Remnant Ablation in Well-Differentiated Thyroid Cancer
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Reconstitution, Preparation, and Administration of THYROGEN
2.3 Timing of Serum Thyroglobulin Testing Following THYROGEN Administration
2.4 Timing for Remnant Ablation and Diagnostic Scanning Following THYROGEN Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 THYROGEN-Induced Hyperthyroidism
5.2 Stroke
5.3 Sudden Rapid Tumor Enlargement
5.4 Risks Associated with Radioiodine Treatment
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Clinical Trials of THYROGEN as an Adjunctive Diagnostic Tool for Well-Differentiated Thyroid Cancer
14.2 Clinical Trials of THYROGEN as an Adjunct for Thyroid Remnant Ablation in Well-Differentiated Thyroid Cancer
14.3 Quality of Life
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Adjunctive Diagnostic Tool for Well-Differentiated Thyroid Cancer
THYROGEN® is indicated for use as an adjunctive diagnostic tool for serum thyroglobulin (Tg) testing with or without radioiodine imaging in the follow-up of patients with well-differentiated thyroid cancer who have previously undergone thyroidectomy.
Limitations of Use:
- THYROGEN-stimulated Tg levels are generally lower than, and do not correlate with, Tg levels after thyroid hormone withdrawal [see Clinical Studies (14.1)].
- Even when THYROGEN-stimulated Tg testing is performed in combination with radioiodine imaging, there remains a risk of missing a diagnosis of thyroid cancer or of underestimating the extent of disease.
- Anti-Tg antibodies may confound the Tg assay and render Tg levels uninterpretable [see Clinical Studies (14.1)]. Therefore, in such cases, even with a negative or low-stage THYROGEN radioiodine scan, consideration should be given to further evaluating patients.
1.2 Adjunct for Thyroid Remnant Ablation in Well-Differentiated Thyroid Cancer
THYROGEN is indicated for use as an adjunctive treatment for radioiodine ablation of thyroid tissue remnants in patients who have undergone a near-total or total thyroidectomy for well-differentiated thyroid cancer and who do not have evidence of distant metastatic thyroid cancer.
Limitations of Use:
- The effect of THYROGEN on thyroid cancer recurrence greater than five years post-remnant ablation has not been evaluated [see Clinical Studies (14.2)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
THYROGEN should be used by physicians knowledgeable in the management of patients with thyroid cancer.
THYROGEN is indicated as a two-injection regimen. The recommended dosage of THYROGEN is a 0.9 mg intramuscular injection to the buttock followed by a second 0.9 mg intramuscular injection to the buttock 24 hours later.
THYROGEN should be administered intramuscularly only. THYROGEN should not be administered intravenously.
Pretreatment with glucocorticoids should be considered for patients in whom tumor expansion may compromise vital anatomic structures [see Warnings and Precautions (5.3)].
Routine measurement of serum TSH levels is not recommended after THYROGEN use.
2.2 Reconstitution, Preparation, and Administration of THYROGEN
The supplied lyophilized powder must be reconstituted with Sterile Water for Injection, USP. THYROGEN should be prepared, and administered in the following manner:
- Reconstitute each 0.9 mg vial of THYROGEN with 1.2 mL of Sterile Water for Injection, USP to yield a single-dose solution containing 0.9 mg/mL of thyrotropin alfa that delivers 1 mL (0.9 mg).
- Gently swirl the contents of the vial until all the material is dissolved. Do not shake the solution.
- Visually inspect the reconstituted solution for particulate matter and discoloration prior to administration. The reconstituted THYROGEN solution should be clear and colorless. Do not use if the solution has particulate matter or is cloudy or discolored.
- Withdraw 1 mL of the reconstituted THYROGEN solution (0.9 mg of thyrotropin alfa) and inject intramuscularly in the buttocks. Discard any unused portions.
- The reconstituted THYROGEN solution must be injected within 3 hours unless refrigerated.
- If necessary, the reconstituted solution can be stored refrigerated at a temperature between 2°C and 8°C (36°F to 46°F) for up to 24 hours, while avoiding microbial contamination.
- Do not mix with other substances.
2.3 Timing of Serum Thyroglobulin Testing Following THYROGEN Administration
For serum thyroglobulin testing, the serum sample should be obtained 72 hours after the final injection of THYROGEN [see Clinical Studies (14.1)].
2.4 Timing for Remnant Ablation and Diagnostic Scanning Following THYROGEN Administration
Oral radioiodine should be given 24 hours after the second injection of THYROGEN in both remnant ablation and diagnostic scanning. The activity of 131I is carefully selected at the discretion of the nuclear medicine physician.
Diagnostic scanning should be performed 48 hours after the radioiodine administration.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 THYROGEN-Induced Hyperthyroidism
When given to patients who have substantial thyroid tissue still in situ or functional thyroid cancer metastases, THYROGEN is known to cause a transient (over 7 to 14 days) but significant rise in serum thyroid hormone concentration. There have been reports of death in non-thyroidectomized patients and in patients with distant metastatic thyroid cancer in which events leading to death occurred within 24 hours after administration of THYROGEN. Patients with residual thyroid tissue at risk for THYROGEN-induced hyperthyroidism include the elderly and those with a known history of heart disease. Hospitalization for administration of THYROGEN and postadministration observation in patients at risk should be considered.
5.2 Stroke
There are postmarketing reports of radiologically-confirmed stroke and neurological findings suggestive of stroke unconfirmed radiologically (e.g., unilateral weakness) occurring within 72 hours (range 20 minutes to three days) of THYROGEN administration in patients without known central nervous system metastases. The majority of such patients were young women taking oral contraceptives at the time of their event or had other risk factors for stroke, such as smoking or a history of migraine headaches. The relationship between THYROGEN administration and stroke is unknown. Patients should be well-hydrated prior to treatment with THYROGEN.
5.3 Sudden Rapid Tumor Enlargement
Sudden, rapid and painful enlargement of residual thyroid tissue or distant metastases can occur following treatment with THYROGEN. This may lead to acute symptoms, which depend on the anatomical location of the tissue. Such symptoms include acute hemiplegia, hemiparesis, and loss of vision one to three days after THYROGEN administration. Laryngeal edema, pain at the site of distant metastasis, and respiratory distress requiring tracheotomy have also been reported after THYROGEN administration.
Pretreatment with glucocorticoids should be considered for patients in whom tumor expansion may compromise vital anatomic structures.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to THYROGEN in 481 thyroid cancer patients who participated in a total of 6 clinical trials of THYROGEN: 4 trials for diagnostic use and 2 trials for ablation. In clinical trials, patients had undergone near-total thyroidectomy and had a mean age of 46.1 years. Thyroid cancer diagnosis was as follows: papillary (69.2%), follicular (12.9%), Hurthle cell (2.3%) and papillary/follicular (15.6%). Most patients received 2 intramuscular injections of 0.9 mg of THYROGEN injection gin 24 hours apart [see Clinical Studies (14.1, 14.2)].
The safety profile of patients who have undergone thyroidectomy and received THYROGEN as adjunctive treatment for radioiodine ablation of thyroid tissue remnants for well-differentiated thyroid cancer did not differ from that of patients who received THYROGEN for diagnostic purposes.
Reactions reported in ≥1% of patients in the combined trials are summarized in Table 1. In some studies, an individual patient may have participated in both THYROGEN and thyroid hormone withdrawal [see Clinical Studies (14.1, 14.2)].
Table 1: Summary of Adverse Reactions by THYROGEN and Thyroid Hormone Withdrawal in Pooled Clinical Trials (≥1% of Patients in any Phase) THYROGEN Thyroid Hormone Withdrawal (N=481) (N=418) Preferred Term n (%) n (%) Nausea 53 (11) 2 (<1) Headache 29 (6) 0 Fatigue 11 (2) 2 (<1) Vomiting 11 (2) 0 Dizziness 9 (2) 0 (0.0) Asthenia 5 (1) 1 (<1) 6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of THYROGEN. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Transient (<48 hours) influenza-like symptoms, including fever (>100°F/38°C), chills/shivering, myalgia/arthralgia, fatigue/asthenia/malaise, headache, and chills.
- Hypersensitivity including urticaria, rash, pruritus, flushing, and respiratory signs and symptoms.
- Injection site reactions, including pain, erythema, bruising, and pruritus.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
THYROGEN may be used in combination with radioiodine (RAI). If THYROGEN is administered with RAI, the combination regimen is contraindicated in pregnant women because fetal exposure to RAI can lead to neonatal hypothyroidism, which in some cases is severe and irreversible. Refer to the RAI prescribing information for more information on use during pregnancy.
Available data from case reports and postmarketing experience with THYROGEN use in pregnant women are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Animal reproduction studies have not been conducted with THYROGEN.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
The concomitant use of THYROGEN and therapeutic radioiodine (RAI) is contraindicated in lactating women because RAI concentrates in the breast tissue and increases the risk of radiation breast toxicity (refer to the therapeutic RAI Prescribing Information).
If THYROGEN is administered with RAI for diagnostic use, discontinue breastfeeding after RAI administration because of the potential for serious adverse reactions from RAI in the breastfed infant (refer to the diagnostic RAI Prescribing Information).
If THYROGEN is not administered with RAI, the developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for THYROGEN and any potential adverse effects on the breastfed child from THYROGEN or from the underlying maternal condition.
There are no available data on the presence of thyrotropin alfa in human milk, the effects on the breastfed infant, or the effects on milk production.
8.3 Females and Males of Reproductive Potential
THYROGEN may be used in combination with radioiodine (RAI). If THYROGEN is administered with RAI, the information for RAI regarding pregnancy testing, contraception, and infertility also applies to the combination regimen. Refer to the RAI prescribing information for additional information.
8.5 Geriatric Use
In pooled clinical studies of THYROGEN, 60 patients (12%) were >65 years, and 421 (88%) were ≤65 years of age. Results from controlled trials do not indicate a difference in the safety and efficacy of THYROGEN between adult patients less than 65 years and those over 65 years of age [see Warnings and Precautions (5.1)].
-
10 OVERDOSAGE
In clinical trials of THYROGEN, three patients experienced symptoms after receiving THYROGEN doses higher than those recommended. Two patients had nausea after a 2.7 mg IM dose (3 times the recommended dose), and in one of these patients, the event was accompanied by weakness, dizziness and headache. Another patient experienced nausea, vomiting and hot flashes after a 3.6 mg IM dose (4 times the recommended dose). There is no specific therapy for THYROGEN overdose. Supportive care is recommended.
-
11 DESCRIPTION
Thyrotropin alfa, a recombinant human thyroid stimulating hormone, is a heterodimeric glycoprotein comprised of two non-covalently linked subunits, an alpha subunit of 92 amino acid residues containing two N-linked glycosylation sites and a beta subunit of 118 residues containing one N-linked glycosylation site. The amino acid sequence of thyrotropin alfa is identical to that of human pituitary TSH. Thyrotropin alfa is synthesized in a genetically modified Chinese hamster ovary cell line.
Both thyrotropin alfa and naturally occurring human pituitary TSH are synthesized as a mixture of glycosylation variants. Unlike pituitary TSH, which is secreted as a mixture of sialylated and sulfated forms, thyrotropin alfa is sialylated but not sulfated. The biological activity of thyrotropin alfa is determined by a cell-based bioassay. In this assay, cells expressing a functional TSH receptor and a cAMP-responsive element coupled to a heterologous reporter gene, luciferase, enable the measurement of thyrotropin alfa activity by measuring the luciferase response. The specific activity of thyrotropin alfa is determined relative to an internal Genzyme reference standard that was calibrated against the World Health Organization (WHO) human TSH reference standard.
THYROGEN (thyrotropin alfa) for injection is a sterile, white to off-white lyophilized powder in a single-dose vial for intramuscular use after reconstitution.
Each single-dose vial provides 0.9 mg of thyrotropin alfa, and contains mannitol (36 mg); sodium chloride (2.4 mg);sodium phosphate dibasic, heptahydrate (3.7 mg); and sodium phosphate monobasic, monohydrate (1.4 mg). After reconstitution with 1.2 mL of Sterile Water for Injection, USP, the concentration is 0.9 mg/mL with a deliverable volume of 1 mL (0.9 mg) and a pH of approximately 6.5 to 7.5.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Thyrotropin (TSH) is a pituitary hormone that stimulates the thyroid gland to produce thyroid hormone. Binding of thyrotropin alfa to TSH receptors on normal thyroid epithelial cells or on well-differentiated thyroid cancer tissue stimulates iodine uptake and organification, and synthesis and secretion of thyroglobulin (Tg), triiodothyronine (T3) and thyroxine (T4).
The effect of thyroid stimulating hormone activation of thyroid cells is to increase uptake of radioiodine to allow scan detection or radioiodine killing of thyroid cells. TSH activation also leads to the release of thyroglobulin by thyroid cells. Thyroglobulin functions as a tumor marker which is detected in blood specimens.
12.3 Pharmacokinetics
The pharmacokinetics of THYROGEN was studied in 16 patients with well-differentiated thyroid cancer given a single 0.9 mg IM dose. Mean peak serum TSH concentrations of 116±38 mU/L were reached between 3 and 24 hours after injection (median of 10 hours). The mean apparent elimination half-life was 25±10 hours. The organ(s) of TSH clearance in man have not been identified, but studies of pituitary-derived TSH suggest the involvement of the liver and kidneys.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term toxicity studies in animals have not been performed with THYROGEN to evaluate the carcinogenic potential of the drug. THYROGEN was not mutagenic in the bacterial reverse mutation assay. Studies have not been performed with THYROGEN to evaluate the effects on fertility.
-
14 CLINICAL STUDIES
14.1 Clinical Trials of THYROGEN as an Adjunctive Diagnostic Tool for Well-Differentiated Thyroid Cancer
Two prospective, randomized phase 3 clinical trials were conducted in patients with well-differentiated thyroid cancer to compare 131I whole body scans obtained after THYROGEN injection to 131I whole body scans after thyroid hormone withdrawal. A cross-over, non-blinded design was used in both trials. Oral radioiodine was given 24 hours after the second injection of THYROGEN, and scanning was done 48 hours after the radioiodine administration. Each patient was scanned first following THYROGEN and then scanned after thyroid hormone withdrawal. In both studies, the primary endpoint was the rate of concordant scans (scan findings in agreement in a given patient using each preparation method).
Study 1 (n=127) compared the diagnostic scanning following a THYROGEN regimen of 0.9 mg IM daily on two consecutive days to thyroid hormone withdrawal. In addition to body scans, Study 2 (n=229) also compared thyroglobulin (Tg) levels obtained after THYROGEN to those at baseline and to those after thyroid hormone withdrawal. All Tg testing was performed in a central laboratory using a radioimmunoassay (RIA) with a functional sensitivity of 2.5 ng/mL. Patients who were included in the Tg analysis were those who had undergone total or near-total thyroidectomy with or without 131I ablation, had <1% uptake in the thyroid bed on a scan after thyroid hormone withdrawal, and did not have detectable anti-Tg antibodies. The maximum THYROGEN Tg value was obtained 72 hours after the final THYROGEN injection, and this value was used in the analysis.
Diagnostic Radioiodine Whole Body Scan Results
Study 1 enrolled 127 patients, 71% were female and 29% male, and mean age was 44 years. The study included the following forms of differentiated thyroid cancer: papillary cancer (88%), follicular cancer (9%), and Hurthle cell (2%). Study results are displayed in Table 2.
In Study 2, patients with differentiated thyroid cancer who had been thyroidectomized (n=229) were randomized into one of two THYROGEN treatment regimens: THYROGEN 0.9 mg IM daily on two consecutive days (n=117), and THYROGEN 0.9 mg IM daily on days 1, 4 and 7 (n=112). Each patient was scanned first using THYROGEN, then scanned using thyroid hormone withdrawal. The group receiving the THYROGEN 0.9 mg IM × 2 regimen was 63% female/27% male, had a mean age of 44 years, and generally had low-stage papillary or follicular cancer (AJCC/TNM Stage I 61%, Stage II 19%, Stage III 14%, Stage IV 5%). The group receiving the THYROGEN 0.9 mg IM × 3 regimen was 66% female/34% male, had a mean age of 50 years, and generally had low-stage papillary or follicular cancer (AJCC/TNM Stage I 50%, Stage II 20%, Stage III 20%, Stage IV 9%). The amount of radioiodine used for scanning was 4 mCi ± 10%, and scanning times were lengthened in some patients to capture adequate images (30 minute scans, or 140,000 counts). Scan pairs were assessed by blinded readers. Study results are presented in Table 2.
Table 2: Concordance of Positive Thyroid Scans Following THYROGEN Treatment with Scans Following Thyroid Hormone Withdrawal Number of scan pairs by disease category Concordance of scan pairs between THYROGEN scan and thyroid hormone withdrawal scan - *
- Across both studies uptake was detected on the THYROGEN scan but not observed on the scan after thyroid hormone withdrawal in 5 patients with remnant or cancer in the thyroid bed.
- †
- In the two clinical studies radioiodine scan results using thyroid hormone withdrawal were taken as the true clinical status of each patient and as the comparator for THYROGEN scans. Thyroid hormone withdrawal trace-positive scans were scored conservatively as positive with no allowance for false positives.
Study 1 (0.9 mg IM qd × 2) Positive for remnant or cancer in thyroid bed 48 81% Positive for metastatic disease 15 73% Total positive withdrawal scans*,† 63 79% Study 2 (0.9 mg IM qd × 2) Positive for remnant or cancer in thyroid bed 35 86% Positive for metastatic Disease 9 67% Total positive withdrawal scans*,† 44 82% Across the two clinical studies, and scoring all false positives in favor of thyroid hormone withdrawal, the majority of positive scans using THYROGEN and thyroid hormone withdrawal were concordant. The THYROGEN scan failed to detect remnant and/or cancer localized to the thyroid bed in 17% (14/83) of patients in whom it was detected by a scan after thyroid hormone withdrawal. In addition, the THYROGEN scan failed to detect metastatic disease in 29% (7/24) of patients in whom it was detected by a scan after thyroid hormone withdrawal.
Thyroglobulin (Tg) Results
THYROGEN Tg testing alone and in combination with diagnostic whole body scanning: comparison with results after thyroid hormone withdrawal
In anti-Tg antibody negative patients with a thyroid remnant or cancer (as defined by a withdrawal Tg ≥2.5 ng/mL or a positive scan [after thyroid hormone withdrawal or after radioiodine therapy]), the THYROGEN Tg was positive (≥2.5 ng/mL) in 69% (40/58) of patients after 2 doses of THYROGEN.
In these same patients, adding the whole body scan increased the detection rate of thyroid remnant or cancer to 84% (49/58) of patients after 2 doses of THYROGEN.
Among patients with metastatic disease confirmed by a post-treatment scan or by lymph node biopsy (35 patients), THYROGEN Tg was positive (≥2.5 ng/mL) in all 35 patients, while Tg on thyroid hormone suppressive therapy was positive (≥2.5 ng/mL) in 79% of these patients.
As with thyroid hormone withdrawal, the intra-patient reproducibility of THYROGEN testing with regard to both Tg stimulation and radioiodine imaging has not been studied.
Hypothyroid Signs and Symptoms
THYROGEN administration was not associated with the signs and symptoms of hypothyroidism that accompanied thyroid hormone withdrawal as measured by the Billewicz scale. Statistically significant worsening in all signs and symptoms were observed during the hypothyroid phase (p<0.01) (Figure 1).
Figure 1: Hypothyroid Symptom Assessment Billewicz Scale Diagnostic Indication 0.9 mg THYROGEN q 24 hours × 2 doses vs Thyroid Hormone Withdrawal Phase
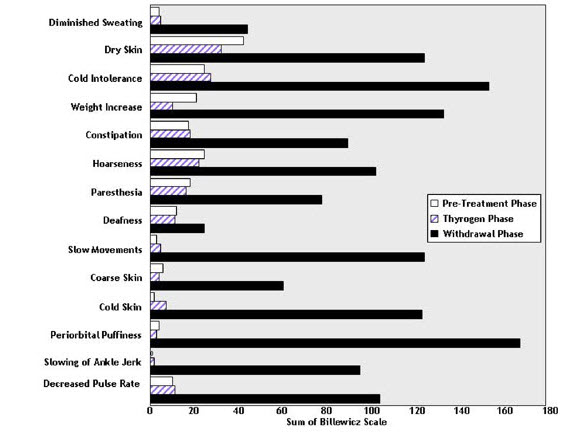
14.2 Clinical Trials of THYROGEN as an Adjunct for Thyroid Remnant Ablation in Well-Differentiated Thyroid Cancer
A randomized, prospective clinical trial compared the rates of thyroid remnant ablation achieved after preparation of patients with thyroid hormone withdrawal or THYROGEN. Patients (n=63) with low-risk, well-differentiated thyroid cancer who underwent near-total thyroidectomy were made euthyroid after surgery by receiving thyroid hormone replacement and were subsequently randomized to a thyroid hormone withdrawal or THYROGEN. Patients in the THYROGEN group received THYROGEN 0.9 mg IM daily on 2 consecutive days and radioiodine 24 hours after the second dose of THYROGEN. Patients in the thyroid hormone withdrawal group had the thyroid replacement withheld until they became hypothyroid. Patients in both groups received 100 mCi 131I ± 10% with the intent to ablate any thyroid remnant tissue. The primary endpoint of the study was the rate of successful ablation, and was assessed 8 months later by a THYROGEN-stimulated radioiodine scan. Patients were considered successfully ablated if there was no visible thyroid bed uptake on the scan, or if visible, uptake was less than 0.1%. Table 3 summarizes the results of this evaluation.
Table 3: Remnant Ablation in Clinical Trial of Patients with Well-Differentiated Thyroid Cancer Group* Mean Age
(Yr)Gender
(F:M)Cancer Type
(Pap:Fol)Ablation Criterion
(Measure at 8 Months)Thyroid Bed Activity <0.1% No Visible Thyroid Bed Activity† Abbreviations: fol = follicular, pap = papillary Thyroid Hormone Withdrawal
(N=28)43 24:6 29:1 28/28 (100%) 24/28 (86%) THYROGEN
(N=32)44 26:7 30:3 32/32 (100%) 24/32 (75%) The mean radiation dose to blood was 0.266 ± 0.061 mGy/MBq in the THYROGEN group and 0.395 ± 0.135 mGy/MBq in the thyroid hormone withdrawal group. Radioiodine residence time in remnant tissue was 0.9 ± 1.3 hours in the THYROGEN group and 1.4 ± 1.5 hours in the thyroid hormone withdrawal group. It is not known whether this difference in radiation exposure would convey a clinical benefit.
Patients who completed were followed up for a median duration of 3.7 years (range 3.4 to 4.4 years) following radioiodine ablation. Tg testing was also performed. The main objective of the follow-up study was to evaluate the status of thyroid remnant ablation by using THYROGEN-stimulated neck imaging. Of the fifty-one patients enrolled, forty-eight patients received THYROGEN for remnant neck/whole body imaging and/or thyroglobulin testing. Only 43 patients had imaging. Patients were still considered to be successfully ablated if there was no visible thyroid bed uptake on the scan, or if visible, uptake was less than 0.1%. All patients from both original treatment groups who had scanning were found to still be ablated. Of 37 patients who were Tg-antibody negative, 16/17 (94%) of patients in the former thyroid hormone withdrawal group and 19/20 (95%) of patients in the former THYROGEN group maintained successful ablation measured as stimulated serum Tg levels of <2 ng/mL.
No patient had a definitive cancer recurrence during the 3.7 years of follow-up. Overall, 48/51 patients (94%) had no evidence of cancer recurrence, 1 patient had possible cancer recurrence (although it was not clear whether this patient had a true recurrence or persistent tumor from the regional disease noted at the start of the initial study), and 2 patients could not be assessed.
Two large prospective multicenter randomized studies compared THYROGEN to thyroid hormone withdrawal using two different doses of radioactive iodine in patients with differentiated thyroid cancer who had been thyroidectomized. In both studies, patients were randomized to 1 of 4 treatment groups: THYROGEN + 30 mCi 131I, THYROGEN + 100 mCi 131I, thyroid hormone withdrawal + 30 mCi 131I, or thyroid hormone withdrawal + 100 mCi 131I. Patients were assessed for efficacy (ablation success rates) at approximately 8 months.
The first study (Study A) randomized 438 patients (tumor stages T1–T3, Nx, N0 and N1, M0). Ablation success was defined as radioiodine uptake of <0.1% in the thyroid bed and stimulated thyroglobulin levels of <2.0 ng/mL. Results are summarized below (Table 4).
Table 4: Remnant Ablation Rates in Study A THYROGEN Thyroid Hormone Withdrawal Total 95% CI of difference in ablation rate (low dose minus high dose): -10.2% to 2.6%
95% CI of difference in ablation rate (THYROGEN - Thyroid Hormone Withdrawal): -6.0% to 6.8%Low-dose radioiodine 91/108
(84.3%)91/106
(85.8%)182/214
(85.0%)High-dose Radioiodine 92/102
(90.2%)92/105
(87.6%)184/207
(88.9%)Total 183/210
(87.1%)183/211
(86.7%)366/421
(86.9%)For Study A, 434 (99%) of the original 438 patients were followed up for disease recurrence. The median follow-up was 6.5 years (0.03 to 10.6 years).
The second study (Study B) randomized 752 patients with low-risk thyroid cancer (tumor stages pT1 <1 cm and N1 or Nx, pT1 >1–2 cm and any N stage, or pT2 N0, all patients M0). Ablation success was defined by neck ultrasound and stimulated thyroglobulin of ≤1.0 ng/mL. Results are summarized below (Table 5).
Table 5: Remnant Ablation Rates in Study B THYROGEN Thyroid Hormone Withdrawal Total 95% CI of difference in ablation rate (low dose minus high dose): -5.8% to 0.9%
95% CI of difference in ablation rate (THYROGEN minus Thyroid Hormone Withdrawal): -4.5% to 2.2%Low-dose radioiodine 160/177
(90.4%)156/170
(91.8%)316/347
(91.1%)High-dose Radioiodine 159/171
(93.0%)156/166
(94.0%)315/337
(93.5%)Total 319/348
(91.6%)312/336
(92.9%)631/684
(92.3%)For Study B, 726 (97%) of the original 752 patients were followed up for disease recurrence. The median follow-up was 5.4 years (0.5 to 9.2 years).
Five-year follow-up data of THYROGEN for remnant ablation with two different RAI doses in Study A and Study B observed similar rates of thyroid cancer recurrence as thyroid hormone withdrawal.
14.3 Quality of Life
Quality of Life (QOL) was measured during both the diagnostic study [see Clinical Studies (14.1)] and the ablation of thyroid remnant study [see Clinical Studies (14.2)] using the SF-36 Health Survey, a standardized, patient-administered instrument assessing QOL across eight domains measuring both physical and mental functioning. In the diagnostic study and in the remnant ablation study, following THYROGEN administration, little change from baseline was observed in any of the eight QOL domains of the SF-36. Following thyroid hormone withdrawal in the diagnostic study, statistically significant negative changes were noted in all eight QOL domains of the SF-36. The difference between treatment groups was statistically significant (p<0.0001) for all eight QOL domains, favoring THYROGEN over thyroid hormone withdrawal (Figure 2). In the remnant ablation study, following thyroid hormone withdrawal, statistically significant negative changes were noted in five of the eight QOL domains (physical functioning, role physical, vitality, social functioning and mental health).
Figure 2: SF-36 Health Survey Results Quality of Life Domains Diagnostic Indication
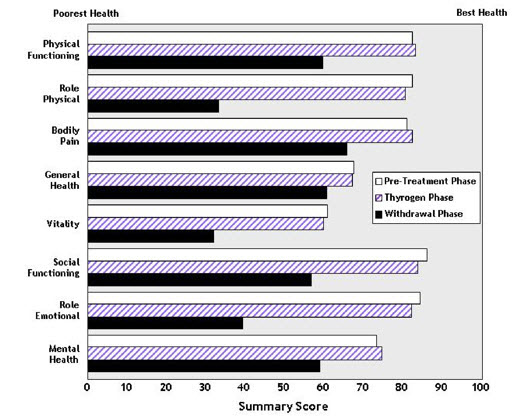
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Adverse Reactions
- Inform patients that the most common adverse events from clinical experience were nausea and headache.
- Advise patients to seek immediate medical attention should they experience severe symptoms.
Important Information
- Prior to THYROGEN administration, counsel patients to seek care immediately for any neurologic symptoms occurring after administration of the drug.
- Inform patients for whom THYROGEN induced hyperthyroidism could have serious consequences, hospitalization for administration of THYROGEN and postadministrative observation should be considered.
Dosing and Administration
- Patients should be instructed that THYROGEN is for intramuscular administration into the buttock only. THYROGEN should not be administered intravenously.
- Inform patients the treatment regimen is two doses of THYROGEN administered at a 24 hour interval.
- Encourage patients to remain hydrated prior to treatment with THYROGEN.
Schedule of Procedures
- Inform patients that if diagnostic scanning will be performed, radioiodine will be given 24 hours after the second injection of THYROGEN, and patients should return for the scan 48 hours after radioiodine administration.
- Inform patients that if serum Tg testing is performed, blood will be drawn 72 hours or later after the second injection of THYROGEN.
- Inform patients that if remnant ablation is performed radioiodine will be administered 24 hours after the second injection of THYROGEN.
Pregnancy and Lactation Risks Associated with Radioiodine Treatment
- When THYROGEN is administered in combination with radioiodine (RAI), refer to the RAI prescribing information for patient counseling information. Inform patients to notify their healthcare provider immediately in the event of a pregnancy [see Warnings and Precautions (5.4) and Use in Specific Populations (8.1, 8.3)].
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 2 Vial Carton
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
THYROGEN
thyrotropin alfa injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:58468-0030 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength THYROTROPIN ALFA (UNII: AVX3D5A4LM) (THYROTROPIN ALFA - UNII:AVX3D5A4LM) THYROTROPIN ALFA 0.9 mg in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) 30 mg in 1 mL SODIUM PHOSPHATE (UNII: SE337SVY37) 4.25 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 2 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58468-0030-2 2 in 1 CARTON 11/30/1998 1 NDC:58468-0030-1 1 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA020898 11/30/1998 THYROGEN
thyrotropin alfa kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:58468-1849 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58468-1849-4 1 in 1 CARTON 11/30/1998 05/30/2019 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 VIAL 2 mL Part 2 2 VIAL 20 mL Part 1 of 2 THYROGEN
thyrotropin alfa injection, powder, for solutionProduct Information Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength THYROTROPIN ALFA (UNII: AVX3D5A4LM) (THYROTROPIN ALFA - UNII:AVX3D5A4LM) THYROTROPIN ALFA 0.9 mg in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) 30 mg in 1 mL SODIUM PHOSPHATE (UNII: SE337SVY37) 4.25 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 2 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA020898 11/30/1998 05/30/2019 Part 2 of 2 THYROGEN
water injection, solutionProduct Information Route of Administration INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 10 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA020898 11/30/1998 05/30/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA020898 11/30/1998 05/30/2019 Labeler - Genzyme Corporation (025322157) Establishment Name Address ID/FEI Business Operations Genzyme Corporation 968278874 MANUFACTURE(58468-0030, 58468-1849) , API MANUFACTURE(58468-0030, 58468-1849) Establishment Name Address ID/FEI Business Operations Genzyme Ireland Limited 985127419 MANUFACTURE(58468-0030, 58468-1849) Establishment Name Address ID/FEI Business Operations Genzyme Corporation 050424395 LABEL(58468-0030, 58468-1849) , PACK(58468-0030, 58468-1849) Establishment Name Address ID/FEI Business Operations Genzyme Corporation 968278916 ANALYSIS(58468-0030, 58468-1849) Establishment Name Address ID/FEI Business Operations Genzyme Corporation 968302658 ANALYSIS(58468-0030, 58468-1849)




