Label: FEXINIDAZOLE tablet
- NDC Code(s): 0024-4512-14, 0024-4512-24
- Packager: Sanofi-Aventis U.S. LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FEXINIDAZOLE TABLETS safely and effectively. See full prescribing information for FEXINIDAZOLE TABLETS.
FEXINIDAZOLE tablets, for oral use
Initial U.S. Approval: 2021RECENT MAJOR CHANGES
Contraindications (4) 9/2024 INDICATIONS AND USAGE
Fexinidazole Tablets is a nitroimidazole antimicrobial, indicated for the treatment of both first-stage (hemolymphatic) and second-stage (meningoencephalitic) human African trypanosomiasis (HAT) due to Trypanosoma brucei gambiense in patients 6 years of age and older and weighing at least 20 kg. (1)
Limitations of Use
Due to the decreased efficacy observed in patients with severe second stage HAT (cerebrospinal fluid white blood cell count (CSF-WBC) >100 cells/µL) due to T. brucei gambiense disease, Fexinidazole Tablets should only be used in these patients if there are no other available treatment options. (1, 5.1)
DOSAGE AND ADMINISTRATION
Administer Fexinidazole Tablets once daily with food each day at about the same time of the day. Do not break or crush tablets. (2.1, 2.2)
Recommended Dosage of Fexinidazole Tablets in Patients 6 years of age and older and weighing at least 20 kg (2.2) Body Weight Type of Dose Daily Dose Number of Tablets Duration of Treatment Greater than or equal to 35 kg Loading dose 1,800 mg 3 4 days Maintenance dose 1,200 mg 2 6 days Greater than or equal to 20 kg to less than 35 kg Loading dose 1,200 mg 2 4 days Maintenance dose 600 mg 1 6 days DOSAGE FORMS AND STRENGTHS
Tablets: 600 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Decreased Efficacy in Severe Human African Trypanosomiasis caused by Trypanosoma brucei gambiense. (1, 5.1)
- QT Interval Prolongation: Prolongation of the QT interval due to Fexinidazole Tablets occurs in a concentration-dependent manner. Avoid use in patients with known prolongation, proarrhythmic conditions, and concomitant use with drugs that prolong the QT interval, those that block cardiac potassium channels, and/or those that induce bradycardia, or are inducers of hepatic CYP450. (5.2, 7.1, 7.2, 12.2)
- Neuropsychiatric Adverse Reactions: Adverse reactions such as agitation, anxiety, abnormal behavior, depression, suicidal ideation, nightmares, hallucination, and personality change have been observed during therapy. Inform patients and their caregivers of the risk. Consider alternative therapy or increased monitoring of the patient, including hospitalization in patients with psychiatric disorders, or if these adverse reactions occur. (5.3)
- Neutropenia: Avoid concomitant use of drugs which may cause neutropenia and monitor leukocyte count periodically. Monitor patients with neutropenia for symptoms or signs of infection. (5.4, 6.1)
- Potential for Hepatotoxicity: Evaluate liver-related laboratory tests at the start and during treatment. (4, 5.5)
- Risk of Disulfiram-like Reactions Due to Concomitant Use with Alcohol: Nitroimidazole-class drugs may cause a disulfiram-like reaction in patients who concurrently consume alcohol. Advise patients to avoid consumption of alcohol during treatment with and for at least 48 hours after completing therapy. (2.1, 5.6)
- Risk of Psychotic Reactions Due to Concomitant Use with Disulfiram: Psychotic reactions have been reported in patients concurrently taking disulfiram and nitroimidazole-class drugs. Avoid use in patients who have taken disulfiram within the last two weeks. (5.7).
ADVERSE REACTIONS
Most common adverse reactions (incidence >10%) are headache, vomiting, insomnia, nausea, asthenia, tremor, decreased appetite, dizziness, hypocalcemia, dyspepsia, back pain, upper abdominal pain, and hyperkalemia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact sanofi-aventis U.S. LLC at 1-800-633-1610 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Recommended Dosage
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Decreased Efficacy in Severe Human African Trypanosomiasis Caused by Trypanosoma brucei gambiense
5.2 QT Interval Prolongation
5.3 Neuropsychiatric Adverse Reactions
5.4 Neutropenia
5.5 Potential for Hepatotoxicity
5.6 Risk of Disulfiram-like Reaction Due to Concomitant Use with Alcohol
5.7 Risk of Psychotic Reactions Due to Concomitant Use with Disulfiram
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Pharmacodynamic Interactions
7.2 Pharmacokinetic Drug Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Fexinidazole Tablets are indicated for the treatment of both the first-stage (hemolymphatic) and second-stage (meningoencephalitic) human African trypanosomiasis (HAT) due to Trypanosoma brucei gambiense in patients 6 years of age and older and weighing at least 20 kg.
Limitations of Use
Due to the decreased efficacy observed in patients with severe second stage HAT (cerebrospinal fluid white blood cell count (CSF-WBC) >100 cells/µL) due to T. brucei gambiense disease, Fexinidazole Tablets should only be used in these patients if there are no other available treatment options [see Warnings and Precautions (5.1)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
- Patients should be closely followed by their healthcare provider during treatment with Fexinidazole Tablets.
- Fexinidazole Tablets must be administered with food [see Dosage and Administration (2.2)].
- Avoid consumption of alcoholic beverages during treatment with Fexinidazole Tablets and for at least 48 hours after completing therapy [see Warnings and Precautions (5.6)].
- If a first event of vomiting occurs after receiving Fexinidazole Tablets, do not re-dose. Administer the next dose the following day using the recommended treatment schedule [see Adverse Reactions (6.1)].
- If a scheduled dose is missed (not taken on the assigned day), normal dosing should resume the following day until the full course (10 days) of treatment has been completed. The clinical consequences of multiple missed doses of Fexinidazole Tablets are not known.
2.2 Recommended Dosage
Administer Fexinidazole Tablets, orally, once daily for a total of 10 days (loading dose plus maintenance dose) with food each day at about the same time of the day. Do not break or crush Fexinidazole Tablets.
The recommended dosage of Fexinidazole Tablets for patients 6 years of age and older is according to body weight as described in Table 1 below.
Table 1: Recommended Dosage of Fexinidazole Tablets in Patients 6 Years of Age and Older and Weighing at Least 20 kg Body weight Type of Dose Recommended* Daily Dose Number of 600 mg Fexinidazole Tablets Daily Duration of Treatment - *
- Administer Fexinidazole Tablets once daily with food each day at about the same time of the day
Greater than or equal to 35 kg Loading dose 1,800 mg 3 4 days Maintenance dose 1,200 mg 2 6 days Greater than or equal to 20 kg to less than 35 kg Loading dose 1,200 mg 2 4 days Maintenance dose 600 mg 1 6 days - 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Fexinidazole Tablets are contraindicated in:
- Patients with known hypersensitivity to Fexinidazole Tablets and/or any nitroimidazole-class drugs (e.g., metronidazole, tinidazole).
- Patients with severe hepatic impairment [see Warnings and Precautions (5.5), Adverse Reactions (6.1), and Use in Specific Populations (8.7)].
- Patients with Cockayne syndrome. Severe irreversible hepatotoxicity/acute liver failure with fatal outcomes have been reported after initiation of metronidazole, another nitroimidazole drug, structurally related to fexinidazole, in patients with Cockayne syndrome [see Adverse Reactions (6.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Decreased Efficacy in Severe Human African Trypanosomiasis Caused by Trypanosoma brucei gambiense
Decreased efficacy was observed in patients treated with Fexinidazole Tablets as compared to nifurtimox-eflornithine combination therapy (NECT)-treated patients in a randomized, comparative open-label study in the subgroup of patients with severe second stage disease, as defined by cerebrospinal fluid white blood cell count (CSF-WBC) >100 cells/µL at baseline [see Clinical Studies (14)]. The 18-month success rate in this subgroup of patients with severe second stage disease was 86.9% with Fexinidazole Tablets compared to 98.7% with NECT with a difference of -11.8%, 95% confidence interval (CI) (-18.3%, -2.1%). All-cause mortality was higher in patients with severe disease treated with Fexinidazole Tablets than in patients treated with NECT through 24 months (7/160 [4.4%] vs 0/78 [0%], treatment difference 4.4%, 95% CI [-0.9%, 8.9%]).
Patients with severe second stage HAT (CSF-WBC >100 cells/µL) due to T. brucei gambiense disease should only be treated with Fexinidazole Tablets if there are no other available treatment options.
5.2 QT Interval Prolongation
Fexinidazole Tablets have been shown to prolong the QT interval in a concentration-dependent manner [see Clinical Pharmacology (12.2)]. Treatment with Fexinidazole Tablets caused an average increase of 19 msec in the QTcF interval. In clinical trials in HAT patients, three (<1%) patients in the fexinidazole group had a QTcF value of >500 ms versus none in the nifurtimox-eflornithine combination therapy (NECT) group.
Avoid use of Fexinidazole Tablets in patients who have:
- QTcF interval greater than 470 msec
- A history of torsade de pointes, congenital long QT syndrome, cardiac arrhythmias, uncompensated heart failure, or family history of sudden death
- Uncorrected hypokalemia
Avoid concomitant administration of Fexinidazole Tablets with other drugs that are known to prolong the QT interval, those that block cardiac potassium channels, and/or those that induce bradycardia [see Drug Interactions (7.1)].
Avoid concomitant administration of Fexinidazole Tablets with drugs that are inducers of hepatic CYP450 as these drugs may significantly increase plasma concentrations of fexinidazole's active metabolites: fexinidazole sulfoxide (M1) and fexinidazole sulfone (M2). M2 plasma concentrations have been associated with increased QT prolongation risks [see Drug Interactions (7.2)].
If patients are, or need to be, treated with drugs known to prolong QTcF interval or to induce bradycardia either do not initiate therapy with Fexinidazole Tablets until such drugs are eliminated from the body (allow a washout period of 5 half-lives for such other drugs), or do not start such drugs until fexinidazole is eliminated from the body (allow a washout period of 7 days for Fexinidazole Tablets).
5.3 Neuropsychiatric Adverse Reactions
Adult patients treated with Fexinidazole Tablets reported a higher percentage of Central Nervous System (CNS) and psychiatric-related adverse reactions than those treated with nifurtimox eflornithine combination therapy (NECT) in a clinical trial [see Adverse Reactions (6.1)]. Increased incidence in insomnia, headache, and tremor was noted in the patients treated with Fexinidazole Tablets compared to NECT. In the same trial, adverse reactions representing mood changes and psychiatric disorders (such as agitation, anxiety, abnormal behavior, depression, nightmares, hallucination, and personality change) were more common in the patients treated with Fexinidazole Tablets compared to the NECT arm. Suicidal ideation has also been observed with Fexinidazole Tablets [see Adverse Reactions (6.1)]. Healthcare providers should inform patients and their caregivers of the risk for neuropsychiatric adverse reactions during treatment with Fexinidazole Tablets. In patients with current or a history of psychiatric disorders, or should such adverse reactions occur, healthcare providers should consider alternative therapy or increased monitoring of the patient, including hospitalization.
5.4 Neutropenia
Neutropenia (absolute neutrophil count less than 1,000 cells/mm3) has been reported in patients receiving Fexinidazole Tablets [see Adverse Reactions (6.1)]. In Trial 1, the adverse reaction occurred in patients with a baseline absolute neutrophil count of less than 5,000 cells/mm3. Avoid concomitant use of drugs which may cause neutropenia and monitor leukocyte count periodically. Carefully monitor patients with neutropenia for fever or other symptoms or signs of infection and treat promptly if such symptoms or signs occur.
5.5 Potential for Hepatotoxicity
Elevations in liver transaminases occurred in less than two percent of patients receiving Fexinidazole Tablets for the treatment of HAT [see Adverse reactions (6.1) and Overdosage (10)]. Evaluate liver-related laboratory tests at the start [see Contraindications (4)] and during treatment with Fexinidazole Tablets. Monitor patients who develop abnormal liver-related laboratory tests during treatment with Fexinidazole Tablets.
5.6 Risk of Disulfiram-like Reaction Due to Concomitant Use with Alcohol
Nitroimidazole-class drugs may cause a disulfiram-like reaction characterized by flushing, rash, weakness, abdominal cramps, nausea, vomiting and headache in patients who concurrently consume alcohol. Advise patients to avoid consumption of alcohol during treatment with Fexinidazole Tablets and for at least 48 hours after completing therapy [see Dosage and Administration (2.1)].
-
6 ADVERSE REACTIONS
The following serious and otherwise important adverse reactions are discussed in greater detail in other sections of labeling:
- Decreased Efficacy in Severe Human African Trypanosomiasis Caused by Trypanosoma brucei gambiense [see Warnings and Precautions (5.1)]
- QT Interval Prolongation [see Warnings and Precautions (5.2)]
- Neuropsychiatric Adverse Reactions [see Warnings and Precautions (5.3)]
- Neutropenia [see Warnings and Precautions (5.4)]
- Potential for Hepatotoxicity [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Fexinidazole Tablets were evaluated for the treatment of HAT due to T. brucei gambiense in three clinical trials, of which one was comparative and two were noncomparative. Trial 1 compared the safety of Fexinidazole Tablets to nifurtimox-eflornithine combination therapy (NECT) in second stage, meningoencephalitic HAT (N=394). Trial 2 enrolled patients with stage 1 hemolymphatic and early stage 2 HAT (N=230), and Trial 3 assessed the safety of fexinidazole in pediatric patients aged 6 years or older with any stage HAT (N=125).
The three trials were primarily conducted in the Democratic Republic of Congo (DRC) and a total of 749 patients received at least one dose of study medication. The patients ranged from 6 to 73 years of age, and 11 were more than 65 years old. Trials 1 and 3 enrolled more males than females (61% and 54% were males, respectively), while the gender distribution was equally balanced in Trial 2. The mean BMI ranged from 16.1 to 19.3 kg/m2 across trials which was consistent with the nutritional status of the study population. Patients with AST/ALT >2 times the upper limit of normal or total bilirubin >1.5 the upper limit of normal were excluded from the trials.
Trial 1 included 264 patients in the fexinidazole treatment arm and 130 patients in the NECT treatment arm. The patients were followed for up to 24 months from the completion of treatment.
Common Adverse Reactions
The most common adverse reactions occurring in >10% of HAT patients (15 years of age and older) receiving Fexinidazole Tablets in Trial 1 were headache, vomiting, insomnia, nausea, asthenia, tremor, decreased appetite, dizziness, hypocalcemia, dyspepsia, back pain, upper abdominal pain, and hyperkalemia.
Selected adverse reactions occurring in ≥2% of HAT patients 15 years of age and older receiving Fexinidazole Tablets in Trial 1 are provided in Table 2.
Table 2: Selected Adverse Reactions Occurring in ≥2% of HAT Patients 15 Years of Age and Older Receiving Fexinidazole Tablets in Trial 1 Adverse Reaction Fexinidazole Tablets
N=264
N (%)NECT
N=130
N (%)- *
- Defined as an absolute neutrophil count of less than 1,000 cells/mm3 occurring at any time following the first dose of study drug to the end of the study.
Blood and lymphatic system disorders Neutropenia* 15 (5.7%) 4 (3.1%) Cardiac disorders Palpitations 13 (4.9%) 5 (3.8%) Eye disorders Photophobia 6 (2.3%) 0 Gastrointestinal disorders Vomiting 75 (28.4%) 37 (28.4%) Nausea 68 (25.8%) 20 (15.4%) Dyspepsia 34 (12.9%) 10 (7.7%) Abdominal pain upper 27 (10.2%) 6 (4.6%) Salivary hypersecretion 16 (6.1%) 3 (2.3%) Constipation 13 (4.9%) 2 (1.5%) Abdominal distension 8 (3.0%) 0 Gastritis 8 (3.0%) 2 (1.5%) General disorders and administration site conditions Asthenia 60 (22.7%) 19 (14.6%) Feeling hot 25 (9.5%) 3 (2.3%) Chest pain 23 (8.7%) 5 (3.8%) Gait disturbance 12 (4.5%) 2 (1.5%) Metabolism and nutrition disorders Decreased appetite 56 (21.2%) 24 (18.5%) Hypocalcemia 36 (13.6%) 3 (2.3%) Hypoalbuminemia 23 (8.7%) 4 (3.1%) Musculoskeletal and connective tissue disorders Back pain 30 (11.4%) 12 (9.2%) Neck pain 23 (8.7%) 7 (5.4%) Muscle Spasms 7 (2.7%) 1 (0.8%) Nervous system disorders Headache 92 (34.8%) 32 (24.6%) Tremor 58 (22.0%) 15 (11.5%) Dizziness 50 (18.9%) 18 (13.8%) Extrapyramidal disorder 9 (3.4%) 2 (1.5%) Paresthesia 6 (2.3%) 0 Psychiatric disorders Insomnia 74 (28.0%) 15 (11.5%) Agitation 10 (3.8%) 1 (0.8%) Anxiety 10 (3.8%) 0 Abnormal behavior 7 (2.7%) 1 (0.8%) Respiratory, thoracic and mediastinal disorders Cough 16 (6.0%) 6 (4.6%) Dyspnea 6 (2.3%) 1 (0.8%) Skin and subcutaneous tissue disorders Pruritus 10 (3.8%) 4 (3.1%) Hyperhidrosis 7 (2.7%) 2 (1.5%) Vascular disorders Hot flush 13 (4.9%) 4 (3.1%) Hypertension 12 (4.5%) 1 (0.8%) Other Adverse Reactions with Fexinidazole Tablets Occurring in Trial 1
The following adverse reactions were reported in less than 2% of patients aged 15 years and older with HAT, treated with Fexinidazole Tablets in Trial 1:
Psychiatric Disorders: hallucinations, psychotic disorder, depression, personality change, suicidal ideation
Laboratory Investigations: elevations of liver transaminases [see Warnings and Precautions (5.5)] and Overdosage (10)]
The safety profile of Fexinidazole Tablets in Trials 2 and 3, including in pediatric subjects aged 6–15 years old, was similar to that of Trial 1 [see Use in Specific Populations (8.4)].
Specific Adverse Reactions
Vomiting
In the clinical trials, the incidence of vomiting within 30 minutes of administration of Fexinidazole Tablets was higher in pediatric patients (20%) than in adult patients (6.1%). There was a trend of increased incidence of vomiting during the loading phase. Generally, vomiting did not lead to treatment discontinuation.
6.2 Postmarketing Experience
The following adverse reaction has been identified and reported during post-approval use of other nitroimidazole agents. Because the reports of this reaction are voluntary and the population is of uncertain size, it is not always possible to reliably estimate the frequency of the reaction or establish a causal relationship to drug exposure.
Metronidazole, Another Nitroimidazole Product, Structurally Related to Fexinidazole: Cases of severe irreversible hepatotoxicity/acute liver failure, including cases with fatal outcomes with very rapid onset after initiation of systemic use of metronidazole, another nitroimidazole agent structurally related to fexinidazole, have been reported in patients with Cockayne syndrome (latency from drug start to signs of liver failure as short as 2 days) [see Contraindications (4)].
-
7 DRUG INTERACTIONS
7.1 Pharmacodynamic Interactions
Herbal Medicines and Supplements
There is a potential for pharmacodynamic interactions and/or toxicities between fexinidazole and herbal medicines and supplements. Avoid concomitant use of herbal medicines and supplements during treatment with Fexinidazole Tablets.
Drugs that May Prolong the QT Interval and/or Induce Bradycardia
Coadministration of Fexinidazole Tablets with drugs known to block potassium channels (e.g., antiarrhythmics, neuroleptics, fluoroquinolones, imidazole and triazole antifungals, pentamidine) prolong the QT interval (e.g., antimalarials, phenothiazines, tricyclic antidepressants, terfenadine and astemizole, IV erythromycin, and quinolone antibacterial drugs) and/or induce bradycardia (such as β-blockers) should be avoided [see Warnings and Precautions (5.2)].
7.2 Pharmacokinetic Drug Interactions
Table 3: Effect of Fexinidazole on other Drugs Drugs Metabolized by Cytochrome P450 (CYP) 3A4/5 Examples (not fully inclusive): Lovastatin, simvastatin, nisoldipine, saquinavir, midazolam, certain hormonal contraceptives (e.g., birth control pills, skin patches, implant) Clinical Impact Fexinidazole is considered a moderate CYP3A4/5 inducer [see Clinical Pharmacology (12.3)]. Co-administration of Fexinidazole Tablets and CYP3A4/5 substrates may reduce the systemic exposures of CYP3A4/5 substrate drug(s), which may lead to reduced efficacy [see Clinical Pharmacology (12.3)]. Prevention or Management Avoid concomitant use with Fexinidazole Tablets. If coadministration cannot be avoided, monitor for lack of efficacy of sensitive substrate drugs. If concomitant use of oral contraceptives cannot be avoided, an alternative contraceptive that is not affected by enzyme inducers (e.g., intrauterine system) or additional nonhormonal contraception (e.g., condoms) is recommended during treatment with Fexinidazole Tablets and for at least 5 days after the last dose. Drugs Metabolized by CYP1A2 or CYP2C19 Examples (not fully inclusive): CYP1A2: duloxetine, tacrine, tizanidine, theophylline
CYP2C19: lansoprazole, mephenytoin, diazepamClinical Impact Increased risk for adverse reactions associated with increased concentrations of the drug due to inhibition of either CYP1A2 or CYP2C19 by fexinidazole [see Clinical Pharmacology (12.3)]. Prevention or Management Monitor for adverse reactions associated with these drugs when used concomitantly with Fexinidazole Tablets. Drugs Metabolized by CYP2B6 Examples (not fully inclusive): Bupropion, efavirenz Clinical Impact Increased risk for the lack of efficacy associated with decreased plasma concentrations of the drug due to induction of CYP2B6 by fexinidazole and Metabolite M1 [see Clinical Pharmacology (12.3)]. Prevention or Management Avoid concomitant use with Fexinidazole Tablets. If coadministration cannot be avoided, monitor for lack of efficacy of these drugs. Drugs Metabolized by uridine 5'-diphospho--glucuronosyl transferase (UGT) Examples (not fully inclusive): Zidovudine, bictegravir, cabotegravir, dolutegravir, bexagliflozin, canagliflozin, sotagliflozin, deferasirox, lamotrigine, selexipag Clinical Impact Fexinidazole metabolites have the potential to be UGT inducers [see Clinical Pharmacology (12.3)]. Co-administration of Fexinidazole Tablets and UGT substrates may reduce the systemic exposures of UGT substrate drug(s), which may lead to reduced efficacy. Prevention or Management When fexinidazole is concomitantly administered with UGT substrates, monitor for lack of efficacy of UGT substrate drugs. Drugs Substrates of OCT2, OAT1, OAT3, MATE1, and MATE2-K Transporters Examples (not fully inclusive): Metformin, dofetilide, adefovir, cefaclor, furosemide Clinical Impact Increased risk for adverse reactions associated with increased concentrations of the drug due to inhibition of these transporters by fexinidazole [see Clinical Pharmacology (12.3)]. Prevention or Management Avoid concomitant use with Fexinidazole Tablets. If coadministration cannot be avoided, monitor for adverse reactions associated with these drugs. Table 4: Effect of other Drugs on Fexinidazole CYP450 Inducers Examples (not fully inclusive): Rifampin, phenytoin, St. John's wort, carbamazepine Clinical Impact Increased risk for adverse reactions associated with increased systemic exposure to the M1 and M2 metabolites of fexinidazole. M2 plasma concentrations have been associated with the increased risk of QT interval prolongation. Prevention or Management Avoid concomitant use with Fexinidazole Tablets [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)]. CYP450 Inhibitors Examples (not fully inclusive): Clarithromycin, itraconazole, voriconazole, erythromycin, fluconazole Clinical Impact Multiple CYP450 enzymes are involved in the metabolism of fexinidazole to its pharmacologically active M1 and M2 metabolites. Although no clinical drug interaction studies were performed with CYP450 inhibitors, the formation of the M1 and M2 metabolites may be decreased. Prevention or Management Avoid concomitant use with Fexinidazole Tablets. If coadministration cannot be avoided, monitor for lack of efficacy of fexinidazole due to potential for decreased plasma concentrations of the M1 and M2 metabolites [see Clinical Pharmacology (12.3)]. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are risks to the mother and fetus associated with untreated HAT due to T. brucei gambiense during pregnancy (see Clinical Considerations). Available data from clinical trials with fexinidazole use in pregnant women are insufficient to evaluate for a drug-associated risk of major birth defects or miscarriage.
There were no effects on prenatal development in embryo-fetal studies where pregnant rats were administered oral fexinidazole during organogenesis at a dose similar to the clinical dose based on AUC comparisons. Effects of fexinidazole on embryo-fetal development were observed in the rat and in the rabbit at doses harmful to the dams only. Exposure of fexinidazole and its metabolites at those maternal toxic doses in rats and rabbits were 2 times and less than 0.02 times the clinical exposure, respectively. In the prenatal and postnatal development study, administration of oral fexinidazole to pregnant rats during organogenesis and through lactation resulted in lower body weights in first generation offspring from dams treated at approximately 1.03 times the clinical exposure based on AUC comparisons.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal Risk
There are adverse effects on maternal and fetal outcomes associated with untreated HAT due to T. brucei gambiense in pregnancy. Disease progression may occur during pregnancy. Pregnant women should be treated for HAT due to T. brucei gambiense during pregnancy to prevent vertical transmission. For timing of treatment during pregnancy, consider the benefits of Fexinidazole Tablets to the mother and the potential risks to the fetus.
Data
Animal data
In the embryo-fetal toxicity studies, pregnant rats were exposed from gestation day (GD) 6 through GD 17. There was no effect on prenatal development in the rat up to the daily dose of 200 mg/kg, similar to the clinical dose based on AUC comparisons.
Maternal toxicity was evidenced by the significantly reduced body weight gain observed at 800 mg/kg. Delayed ossification (sternebrae, metacarpals and caudal vertebrae) and reduced fetal and placental weights were observed in the presence of maternal toxicity.
In the rabbit embryo-fetal development study, pregnant rabbits were exposed from GD 6 to GD 20. Fexinidazole resulted in abortions in the presence of maternal toxicity (reduced food consumption and reduced body weight gain) at doses of 20 mg/kg/day and above, less than 0.02 times the clinical exposures, based on pharmacokinetics comparisons.
In the prenatal and postnatal development study, female rats were exposed from GD 6 to lactation day 21. Lower body weights were reported in F1 pups from dams treated (approximately 1.03 times the clinical exposure based on AUC comparisons) throughout lactation. Sexual maturity showed a minimal delay for both males and females. Postweaning development for behavior and reproductive performance did not indicate any late adverse effect on the progeny.
8.2 Lactation
Risk Summary
There are no data on the presence of fexinidazole in human milk or the effect on milk production. There are no reports of adverse effects to the breastfed child associated with fexinidazole exposure through breastmilk based on a limited number of reported cases. Fexinidazole is present in rat milk (see Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Fexinidazole Tablets and any potential adverse effects on the breastfed child from fexinidazole or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of Fexinidazole Tablets for the treatment of both the first-stage (hemolymphatic) and second-stage (meningoencephalitic) HAT due to Trypanosoma brucei gambiense have been established in pediatric patients aged 6 years and older and weighing at least 20 kg. Use of Fexinidazole Tablets for this indication is supported by evidence from an adequate and well-controlled trial in adults with additional efficacy, pharmacokinetic and safety data in pediatric patients aged 6 years and older [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)].
Pediatric patients may be more sensitive to vomiting. The safety profile for Fexinidazole Tablets in pediatric patients was generally similar to that of adult patients with the exception of more frequent vomiting within 2 hours of administration of Fexinidazole Tablets. Vomiting did not result in permanent treatment discontinuation [see Adverse Reactions (6.1)].
The safety and efficacy of Fexinidazole Tablets have not been established in pediatric patients younger than 6 years old and/or less than 20 kg in body weight.
8.5 Geriatric Use
Of the 619 subjects in the three clinical trials treated with Fexinidazole Tablets for HAT, there were 11 subjects who were 65 years of age or older, and no subjects greater than 75 years of age. There were an insufficient number of elderly subjects to detect differences in safety and/or effectiveness between elderly and younger adult patients.
8.6 Renal Impairment
No dosage adjustment is needed for patients with mild to moderate renal impairment with estimated glomerular filtration rates (eGFR) from 30 mL/min/1.73 m2 to less than or equal to 89 mL/min/1.73 m2 [see Clinical Pharmacology (12.3)]. The pharmacokinetics of fexinidazole in patients with severe renal impairment (eGFR less than 30 mL/min/1.73 m2) is unknown. Avoid the use of Fexinidazole Tablets in patients with severe renal impairment.
8.7 Hepatic Impairment
No dosage adjustment of Fexinidazole Tablets is recommended for patients with mild hepatic impairment (Child-Pugh Class A). Moderate hepatic impairment (Child-Pugh Class B) increases fexinidazole and its active metabolite M1 exposures, which may increase the risk of adverse reactions [see Clinical Pharmacology (12.3)]. Use of Fexinidazole Tablets is contraindicated in patients with severe hepatic impairment [see Contraindications (4) and Warnings and Precautions (5.5)].
-
10 OVERDOSAGE
Randomized, controlled clinical studies were conducted in normal adult male subjects who were administered single or multiple oral doses of fexinidazole of up to 3,600 mg daily for 14 days (not an approved dose). The subjects experienced adverse reactions of increased transaminases, vomiting and panic attack [see Adverse Reactions (6.1)].
Reported symptoms of overdosage in a pediatric HAT patient following ingestion of a higher than recommended dosing regimen in Trial 3 included vomiting over the first 5 days of treatment and increased potassium and decreased calcium levels from Day 11 to Week 9.
There is no specific antidote for Fexinidazole Tablets. Treatment should be supportive with appropriate monitoring.
-
11 DESCRIPTION
Fexinidazole Tablets contain fexinidazole, a nitroimidazole antimicrobial drug for oral use.
The chemical name of fexinidazole is 1-methyl-2-{[4-(methylthio)phenoxy]methyl}-5-nitro-1H-imidazole.
Its molecular formula is C12H13N3O3S and the molecular weight is 279.3 g/mol. The structural formula is:
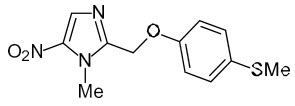
Fexinidazole is a yellow powder. It is practically insoluble in water, sparingly soluble in acetone and acetonitrile, very slightly soluble in ethanol and slightly soluble in methanol.
Fexinidazole 600 mg Tablets contain the active ingredient fexinidazole and the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and sodium lauryl sulfate.
-
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
Cardiac Electrophysiology
Concentration-dependent QTcF prolongation was observed with administration of Fexinidazole Tablets. Based on the exposure-response relationship, the mean (upper 90% confidence interval) increase in QTcF is predicted to be 19.0 msec (23.3 msec) at the recommended dosing regimen. The observed increase in QTcF appears to be associated with the M2 (sulfone) metabolite of fexinidazole [see Warning and Precautions (5.2)].
12.3 Pharmacokinetics
The pharmacokinetics (PK) of fexinidazole and its two pharmacologically active M1 (sulfoxide) and M2 (sulfone) metabolites following administration of the recommended adult dosage regimen of Fexinidazole Tablets in 12 healthy adult male subjects under fed conditions are presented in Table 5.
Table 5: Pharmacokinetics of Fexinidazole and its Active M1 and M2 Metabolites following Administration of Fexinidazole Tablets 1,800 mg Once Daily for 4 Days, Then 1,200 mg Once Daily for 6 Days to Healthy Adult Subjects Under Fed Conditions (N=12) Fexinidazole M1 M2 Cmax = maximum plasma concentration; Tmax = time to maximum concentration; CSF = cerebrospinal fluid; CYP = cytochrome P450 enzymes; AUC0–24 hours = area under the plasma concentration-time curve from time zero to 24 hours, AUC0–t = area under the plasma concentration-time curve from time zero to the last timepoint with measurable analyte concentrations; DBS = dried blood spot NA: Not available or not applicable Mean (±SD) Cmax mcg/mL Day 1 1.6 (±0.4) 8.1 (±2.2) 7.5 (±3.3) Day 4 0.8 (±0.3) 8.0 (±2.3) 19.6 (±5.4) Day 10 0.5 (±0.2) 5.9 (±2.1) 12.5 (±3.5) Mean (±SD) AUC(0–24 hours) mcg∙h/mL Day 1 14.3 (±2.6) 102.3 (±28.5) 110.1 (±41.1) Day 4 11.6 (±2.2) 127.9 (±49.2) 391.5 (±126.7) Day 10 7.0 (±2.5) 84.2 (±36.3) 252.4 (±73.6) Absorption Median Tmax (Range) on Day 4, hours 4 (0–9) 4 (0–6) 6 (0–24) Effect of Food* The AUC of fexinidazole, M1, and M2 were approximately 4 to 5-fold higher following administration with food compared to the fasted state. Distribution Apparent Volume of Distribution on Day 4, L 3222 (±1199) NA NA Plasma Protein Binding 98% 41% 57% Mean (Range) CSF concentrations at 24 hours after the last fexinidazole dose on Day 10, mcg/mL† NA 1.39 (0–4.5) 6.45 (0.3–14.9) Mean (Range) CSF to DBS Ratios† NA 0.53 (0.1–2.2) 0.36 (0.1–0.8) Elimination Mean (±SD) Day 10 Half-life, hours 15 (±6) 16 (±6) 23 (±4) Mean (±SD) Apparent Clearance on Day 4, L/hour 161 (±37) NA NA Metabolism Fexinidazole - Fexinidazole is metabolized to M1 by several CYP450 enzymes, including CYP3A4 and flavin monooxygenases.
Active Metabolites - Several CYP450 enzymes including, CYP3A4 and flavin monooxygenases, are involved in the metabolism of M1 to M2.
- M2 is not further metabolized.
- The AUC0–24 of M1 and M2 are 11 and 34-fold higher, respectively, than that of fexinidazole.
Excretion Urine Less than 3.2% of a given dose of Fexinidazole Tablets, primarily as M1 and M2 metabolites Specific Populations
Elderly patients
No specific pharmacokinetic studies have been performed in patients older than 65 years of age. In a population PK analysis of patients with HAT over a range of ages from 6 to 71 years, age was not a significant covariate affecting the PK of fexinidazole and the M1 and M2 metabolites and no differences in the PK of any of these three moieties were observed.
Pediatric patients
The ranges of plasma AUC values of fexinidazole, M1, and M2 in pediatric and adult HAT patients with body weights greater than or equal to 20 kg were overlapping following administration of Fexinidazole Tablets at the recommended pediatric and adult dosage regimens, indicating similar systemic exposures across body weights of 20 kg and greater.
Hepatic impairment
Following a single 1200 mg dose of Fexinidazole Tablets to subjects with mild hepatic impairment (Child-Pugh A; n=7), fexinidazole, M1 and M2 AUC estimates were 1.40-fold higher, 1.13-fold higher, and 1.20-fold higher, respectively, and their half-life estimates were 1.38-fold higher, 1.18-fold higher, and 0.93-fold lower, respectively. Sum of AUCs of active metabolites M1 and M2 was increased by 5%, in comparison to subjects without hepatic impairment (n=7).
In the same study, in subjects with moderate hepatic impairment (Child-Pugh Class B, n=7), fexinidazole, M1 and M2 AUC estimates were 1.36-fold higher, 1.46-fold higher, and 0.65-fold lower, respectively, and their half-life estimates were 1.77-fold higher, 1.25-fold higher, and 1.34-fold higher, respectively. Sum of AUCs of active metabolites M1 and M2 was decreased by 18%, compared to subjects without hepatic impairment (n=7).
The unbound fraction of fexinidazole was not affected by mild or moderate hepatic impairment. The effect of severe hepatic impairment (Child-Pugh class C) on fexinidazole pharmacokinetics is unknown. [see Contraindications (4) and Use in Specific Populations (8.7)].
Renal Impairment
A population PK analysis, based on baseline renal function, was carried out with data from 317 HAT patients enrolled in clinical trials that included 212 patients with normal renal function (eGFR greater than or equal to 90 mL/min/1.73 m2), 89 patients with mild renal impairment (eGFR 60 to less than 90 mL/min/1.73 m2), and 14 patients with moderate renal impairment (eGFR 30 to less than 60 mL/min/1.73 m2). The predicted AUC0–24 estimates for fexinidazole and its M1 and M2 metabolites were similar in patients with mild or moderate renal impairment compared to those patients without renal impairment. The PK of fexinidazole in patients with severe renal impairment has not been studied [see Use in Specific Populations (8.6)].
Drug Interaction Studies
In vitro studies
Cytochrome P450 (CYP450) and UDP-glucuronosyl transferase (UGT) Enzymes: Fexinidazole has the potential to inhibit CYP1A2, CYP2B6, CYP2C19, CYP2D6, and CYP3A4/5; M1 has the potential to inhibit CYP2C19; M2 does not inhibit any CYPs.
Fexinidazole has the potential to induce CYP1A2 and CYP2B6. Metabolite M1 has the potential to induce CYP1A2, CYP2B6, CYP3A5, UGT1A4, UGT2B4 and UGT2B7. Metabolite M2 has the potential to induce UGT2B4 [see Drug Interactions (7.2)].
Transporter Systems: Fexinidazole inhibits OATP1B1, OATP1B3, OCT2, OAT1, OAT3, MATE1 and MATE2-K transporters. M1 inhibits OAT3, MATE1, and MATE2-K. M2 inhibits OCT2, OAT1, OAT3, MATE1, and MATE2-K. Coadministration with Fexinidazole Tablets may increase the plasma concentrations of drugs that are substrates of these aforementioned transporters [see Drug Interactions (7.2)]. Fexinidazole, M1, or M2 do not inhibit P-gp or BCRP.
Clinical studies
A clinical drug-drug interaction study evaluated the effect of fexinidazole following administration of 1,800 mg Fexinidazole Tablets for four days, followed by 1,200 mg on Day 5, with single dose administration of 100 mg caffeine (probe substrate of CYP1A2) and 20 mg omeprazole (probe substrate of CYP2C19) on Day 4 in healthy subjects. The mean caffeine AUC was 2-fold higher, with no significant increase in Cmax compared to when caffeine was administered alone. The mean Cmax and AUC of omeprazole were approximately 2-fold higher compared to when omeprazole was administered alone [see Drug Interactions (7.2)].
A clinical drug-drug interaction study evaluated the effect of fexinidazole following administration of 1,800 mg Fexinidazole Tablets for four days, followed by 1,200 mg on Day 5 (not an approved dosing regimen), with single dose administration of 2 mg midazolam (probe substrate of CYP3A4/5) on Day 4 in healthy subjects. The mean midazolam AUC was decreased by 57%, mean Cmax by 39% and mean half-life by 33% compared to when midazolam was administered alone [see Drug Interactions (7.2)].
Model-informed approaches
Findings from a static mechanistic model-based analysis predicted that fexinidazole may decrease the systemic PK exposure of CYP2B6 substrates [see Drug Interactions (7.2)].
This model-based analysis predicted no significant drug interaction of fexinidazole with drugs that are substrates of CYP2C8, CYP2C9, or CYP2D6.
12.4 Microbiology
Mechanism of Action
Studies with Trypanosoma brucei and other protozoans suggest that, like for other nitro-containing drugs, the nitroreductase (NTR) enzyme plays an important role in the bioactivation of fexinidazole resulting in generation of reactive amines and damage to DNA and proteins. The activity of fexinidazole and its metabolites (M1 and M2) is trypanocidal and appears to be concentration and time dependent. However, the precise mechanism by which fexinidazole and the two metabolites exhibit activity against T. brucei is not known.
Antimicrobial Activity
Fexinidazole and its two metabolites, M1 and M2, are active against the trypanosomes of Trypanosoma brucei gambiense.
Resistance
In vitro studies suggest a potential for development of resistance in T. brucei against fexinidazole.
The mechanism of resistance appears to be similar to other nitro-containing drugs, such as nifurtimox, and include down-regulation of Type 1 NTR. However, the clinical relevance of these findings is not known.
Cross-Resistance
Nonclinical studies suggest cross-resistance between fexinidazole and other nitro-containing drugs such as nifurtimox. This appears to be due to down regulation of Type I NTR. Although the clinical relevance of these findings is not known, the potential for the development of resistance to fexinidazole in patients previously treated with nifurtimox-eflornithine combination therapy (NECT) cannot be discounted.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
No carcinogenicity study was performed with fexinidazole.
Carcinogenicity has been observed in mice and rats treated chronically with nitroimidazole-class drugs which are structurally similar to fexinidazole. It is unclear if the findings of tumors in lifetime rodent studies indicate a risk to patients taking a 10-day treatment of Fexinidazole Tablets for HAT.
Mutagenesis
Fexinidazole and the M2 metabolite were mutagenic in the Ames test. Fexinidazole was negative in the in vitro micronucleus test in cultured human peripheral blood lymphocytes, the rat liver unscheduled DNA synthesis (UDS) assay, and the in vivo mouse micronucleus assay.
Impairment of Fertility
In the fertility and early embryonic development study, male rats were treated for 28 days prior to start of cohabitation with treated females and throughout the cohabitation period until sacrifice. Female rats were treated for 14 days prior to start of cohabitation with treated males throughout the cohabitation period until copulation occurred and up to GD 7. Fexinidazole showed no effect on fertility parameters and no evidence of impairment of reproductive performance up to the dose of 600 mg/kg/day (estimated to be approximately 1.03 times the clinical exposure based on AUC comparisons).
-
14 CLINICAL STUDIES
Trial 1
The efficacy and safety of Fexinidazole Tablets were evaluated in a randomized, comparative open-label trial (Trial 1, NCT01685827) conducted in adult patients with late second-stage HAT due to T. brucei gambiense. Patients had evidence of parasites in blood, lymph and/or cerebrospinal fluid (CSF) at trial enrollment. If testing for parasites in CSF was negative, a CSF WBC >20 cells/μL was required to confirm late second-stage HAT. Patients (n=394) were randomized in a 2:1 ratio to a 10-day treatment regimen of either Fexinidazole Tablets (n=264) or nifurtimox-eflornithine combination therapy (NECT) (n=130). The mean age was 35 years (range 15 to 71) and 61% were male. The fexinidazole tablet group received 1,800 mg of Fexinidazole Tablets orally once daily on Days 1 through 4, followed by 1,200 mg orally once daily on Days 5 through 10, with all dosing in the fed state. The NECT control arm received nifurtimox tablets 15 mg/kg/day in three divided doses for 10 days as well as eflornithine injectable solution 400 mg/kg/day in two divided doses for 7 days. Patients were hospitalized throughout their treatment and were allowed to leave the hospital from Day 13 onwards if their clinical status was satisfactory. Patients were followed up at 3, 6, 12, 18, and 24 months after the end of treatment visit. HAT symptoms reported at baseline in >50% of patients included headache, pruritus, sleepiness, weight loss, and asthenia. The median CSF WBC count was 157 cells/µL.
The outcome at 18 months was considered a success if patients were classified as a cure or probable cure as defined below:
- Cure: Patient is alive with no evidence of trypanosomes in any body fluid and CSF WBC ≤20 cells/µL.
- Probable cure for patients who refused a lumbar puncture (or who had a hemorrhagic CSF sample) at 18 months: No parasites in the blood or lymph and a satisfactory clinical condition without clinical signs or symptoms (or clinical status is unlikely to be due to HAT), CSF WBC <50 cells/µL at 6 and/or 12 months and not increasing at 12 months, as long as there was no indication of a relapse up to 24 months and no definitive failure (presence of trypanosomes) had been observed before in any body fluid.
Success rates at 18 months are shown in Table 6 for the modified intention-to-treat (mITT) population, which consisted of all randomized patients who received at least one dose of study treatment but excluded 5 randomized patients due to geopolitical unrest. The success rate in the fexinidazole treatment arm was lower than in the NECT arm. Additionally, more deaths occurred in the fexinidazole treatment arm at 24 months (n=9, 3.4%) compared to the NECT treatment arm (n=2, 1.6%). This decreased efficacy and increased mortality was noted in the subgroup of patients who had CSF-WBC >100 cells/µL at baseline [see Warnings and Precautions (5.1)]. The results at 24 months were consistent with the results at 18 months with 24-month success rates of 89.7% (235/262) in the fexinidazole treatment arm and 97.6% (124/127) in the NECT arm.
Table 6: Success Rates at 18 Months (mITT population) in Trial 1 Fexinidazole NECT Difference (97% CI*) - *
- Analysis adjusted for interim analysis to control the overall Type I error at two-sided 0.05.
- †
- Two fexinidazole treated patients were considered as failures due to loss to follow-up and consent withdrawal prior to 18 months.
- ‡
- CSF-WBC represents white blood cell count in cerebrospinal fluid at baseline.
N 262 127 – Success at 18 months† 239 (91.2%) 124 (97.6%) -6.4% (-11.6%, -0.1%) Success at 18 months by baseline CSF-WBC‡ Baseline CSF-WBC ≤100 cells/µL 100/102 (98.0%) 47/49 (95.9%) – Baseline CSF-WBC >100 cells/µL 139/160 (86.9%) 77/78 (98.7%) – Trial 2 and Trial 3
Additional supportive evidence for efficacy in early stage HAT due to T. brucei gambiense, and in pediatric patients was obtained from two single-arm trials: a single-arm trial in adults (Trial 2, NCT02169557), and a single-arm trial in pediatric patients aged 6 to 15 years old and weighing at least 20 kg (Trial 3, NCT02184689). In Trial 2, the mean age of patients was 34 years and 82% of patients had evidence of first-stage HAT (evidence of trypanosomes in the blood or lymph, no trypanosomes in the CSF, and CSF WBC ≤5 cells /µL). In Trial 3, the mean age of patients was 11 years and 55% of patients had evidence of first-stage HAT. Fexinidazole Tablets 1,200 mg, was given in fed condition once a day on Days 1 through 4, followed by 600 mg on Days 5 through 10 to patients weighing <35 kg, and all other patients received the adult dosing regimen. Treatment success proportions in all patients with first- or late-stage HAT were 98.7% (227/230, 95% CI [96.2%, 99.7%]) at 12 months in Trial 2 and 97.6% (122/125, 95% CI [93.1%, 99.5%]) at 12 months in Trial 3. The results at 18 months were consistent with the results at 12 months.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
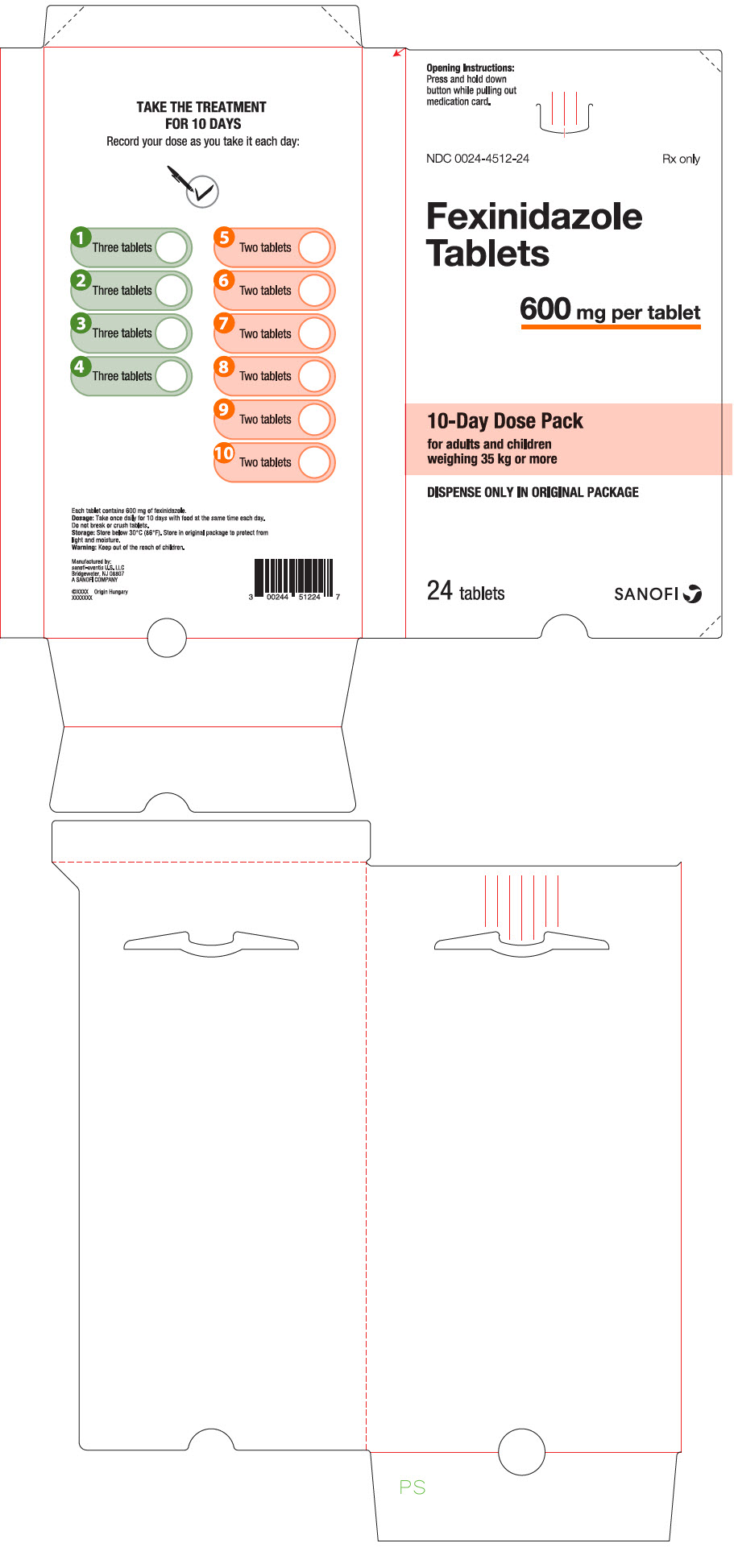
Fexinidazole 600 mg tablets are pale-yellow, round, biconvex tablets debossed with "4512" on one side.
Fexinidazole 600 mg tablets are supplied as:
- NDC 0024-4512-24, child-resistant blister pack of 24 tablets (10-day dose pack) for adults and pediatric patients weighing 35 kg or more.
- NDC 0024-4512-14, child-resistant blister pack of 14 tablets (10-day dose pack) for pediatric patients older than 6 years weighing 20 kg to less than 35 kg.
-
17 PATIENT COUNSELING INFORMATION
Administration with Food
Advise the patient that Fexinidazole Tablets must be taken with food each day at about the same time of the day (e.g., during or immediately after the main meal of the day), to make sure it is adequately absorbed [see Dosage and Administration (2.1, 2.2)].
Alcohol Consumption
Advise patients not to consume alcoholic beverages during treatment with Fexinidazole Tablets and for at least 48 hours after completing Fexinidazole Tablets therapy [see Dosage and Administration (2.1) and Warnings and Precautions (5.6)].
Vomiting
Advise the patient not to administer an additional dose if vomiting occurs after the administration of Fexinidazole Tablets but continue with the next scheduled dose the following day. If a second event of vomiting occurs after administration of any other dose of Fexinidazole Tablets, counsel the patient on the importance of contacting the healthcare provider immediately [see Dosage and Administration (2.1)].
Missed Doses
Advise patients that if a scheduled dose is missed (not taken on the assigned day), normal dosing should resume the following day until the full course (10 days) of treatment has been completed. Counsel the patient on the importance of contacting the health care practitioner immediately if a second scheduled dose is missed [see Dosage and Administration (2.1)].
Neuropsychiatric Adverse Reactions
Counsel patients and their caregivers of the risk for neuropsychiatric adverse reactions, such as insomnia, headache, tremor, mood changes, psychiatric disorders (such as agitation, anxiety, abnormal behavior, depression, nightmares, hallucination, and personality change) and suicidal ideation [see Warnings and Precautions (5.3) and Adverse Reactions (6.1)]. If such adverse reactions occur, advise the patients and their caregivers to contact their healthcare provider immediately.
Dizziness
Counsel the patient that they should not drive or use machines if they feel tired or dizzy. Dizziness, fatigue, asthenia, and somnolence have been reported following treatment with Fexinidazole Tablets [see Adverse Reactions (6.1)].
Drug Interactions
Advise patients to disclose to their healthcare provider all other medications, including herbal medicines, the patient is currently taking while being treated with fexinidazole tablets [see Drug Interactions (7)].
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 600 mg Tablet Dose Pack
-
INGREDIENTS AND APPEARANCE
FEXINIDAZOLE
fexinidazole tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0024-4512 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXINIDAZOLE (UNII: 306ERL82IR) (FEXINIDAZOLE - UNII:306ERL82IR) FEXINIDAZOLE 600 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Product Characteristics Color yellow (pale yellow) Score no score Shape ROUND (biconvex) Size 13mm Flavor Imprint Code 4512 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0024-4512-14 14 in 1 DOSE PACK; Type 0: Not a Combination Product 07/16/2021 2 NDC:0024-4512-24 24 in 1 DOSE PACK; Type 0: Not a Combination Product 07/16/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214429 07/16/2021 Labeler - Sanofi-Aventis U.S. LLC (824676584) Establishment Name Address ID/FEI Business Operations EUROAPI Hungary Ltd. 402388457 analysis(0024-4512) , api manufacture(0024-4512) Establishment Name Address ID/FEI Business Operations Alcami Carolinas Corporation 832395235 manufacture(0024-4512) Establishment Name Address ID/FEI Business Operations Alcami Carolinas Corporation 079646861 pack(0024-4512) , label(0024-4512) Establishment Name Address ID/FEI Business Operations Alcami Carolinas Corporation 831351445 analysis(0024-4512)