Label: REGRANEX- becaplermin gel
- NDC Code(s): 50484-810-15
- Packager: Smith & Nephew, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use REGRANEX ®gel safely and effectively. See full prescribing information for REGRANEX gel.
REGRANEX (becaplermin) gel, for topical use
Initial U.S. Approval: 1997RECENT MAJOR CHANGES
INDICATIONS AND USAGE
REGRANEX is a human platelet-derived growth factor indicated for the treatment of lower extremity diabetic neuropathic ulcers that extend into the subcutaneous tissue or beyond and have an adequate blood supply. REGRANEX is indicated as an adjunct to, and not a substitute for, good ulcer care practices. ( 1)
Limitations of Use:
- The efficacy of REGRANEX has not been established for the treatment of pressure ulcers and venous stasis ulcers. ( 1)
- The effects of REGRANEX on exposed joints, tendons, ligaments, and bone have not been established in humans. ( 1)
- REGRANEX is not intended to be used in wounds that close by primary intention. ( 1)
DOSAGE AND ADMINISTRATION
- For topical use; not for oral, ophthalmic or intravaginal use. ( 2)
- To calculate the length of REGRANEX to apply, measure the greatest length of the ulcer by greatest width of the ulcer in either inches or centimeters. ( 2)
Formula to Calculate Length of Gel to Be Applied Daily
Tube Size
Formula
15 g Tube
Inches: ulcer length × ulcer width × 0.6
15 g Tube
Centimeters: ulcer length × ulcer width ÷ 4
DOSAGE FORMS AND STRENGTHS
Gel: 0.01% ( 3)
CONTRAINDICATIONS
Known neoplasm(s) at the site(s) of application ( 4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Erythematous rashes occurred in 2% of patients treated with REGRANEX. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch, or Smith & Nephew, Inc. at 1-800-441-8227 .
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 8/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Cancer
5.2 Application Site Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Efficacy in Diabetic Lower Extremity Ulcers
14.2 Lack of Efficacy in Pressure Ulcers and Venous Stasis Ulcers
14.3 Observational Studies to Evaluate Cancer Development and Mortality
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
REGRANEX is indicated for the treatment of lower extremity diabetic neuropathic ulcers that extend into the subcutaneous tissue or beyond and have an adequate blood supply, when used as an adjunct to, and not a substitute for, good ulcer care practices including initial sharp debridement, pressure relief and infection control.
Limitations of Use:
The efficacy of REGRANEX has not been established for the treatment of pressure ulcers and venous stasis ulcers [see Clinical Studies (14.2)]and has not been evaluated for the treatment of diabetic neuropathic ulcers that do not extend through the dermis into subcutaneous tissue [Stage I or II, International Association of Enterostomal Therapy (IAET) staging classification] or ischemic diabetic ulcers.
The effects of becaplermin on exposed joints, tendons, ligaments, and bone have not been established in humans [see Nonclinical Toxicology (13.2)].
REGRANEX is not intended to be used in wounds that close by primary intention.
-
2 DOSAGE AND ADMINISTRATION
REGRANEX is for topical use; it is not for oral, ophthalmic or intravaginal use.
The amount of REGRANEX to be applied depends upon the size of the ulcer area. To calculate the length of gel to apply to the ulcer, measure the greatest length of the ulcer by the greatest width of the ulcer in either inches or centimeters. To calculate the length of gel in inches, use the formula shown below in Table 1, and to calculate the length of gel in centimeters, use the formula shown below in Table 2.
Table 1: Formula to Calculate Length of Gel in Inches to Be Applied Daily INCHES Tube Size Formula 15 g tube length × width × 0.6 Using the calculation, each square inch of ulcer surface will require approximately 2/3 inch length of gel squeezed from a 15 g tube. For example, if the ulcer measures 1 inch by 2 inches, then a 1 1/4 inch length of gel should be used for 15 g tubes (1 × 2 × 0.6 = 1 1/4).
Table 2: Formula to Calculate Length of Gel in Centimeters to Be Applied Daily CENTIMETERS Tube Size Formula 15 g tube length × width ÷ 4 Using the calculations for ulcer size in centimeters, each square centimeter of ulcer surface will require approximately a 0.25 centimeter length of gel squeezed from a 15 g tube. For example, if the ulcer measures 4 cm by 2 cm, then a 2 cm length of gel should be used for a 15 g tube [(4 × 2) ÷ 4 = 2].
The amount of REGRANEX to be applied should be recalculated by the physician or wound caregiver at weekly or biweekly intervals depending on the rate of change in ulcer area. The weight of REGRANEX from 15 g tubes is 0.65 g per inch length and 0.25 g per centimeter length.
To apply REGRANEX, the calculated length of gel should be squeezed on to a clean measuring surface, e.g., wax paper, and measured to the correct length with a ruler. The measured REGRANEX is transferred from the clean measuring surface using an application aid and then spread over the entire cleaned ulcer area to yield a thin continuous layer of approximately 1/16 of an inch thickness. The site(s) of application should then be covered by a saline moistened gauze dressing and left in place for approximately 12 hours. The dressing should then be removed and the ulcer rinsed with saline or water to remove residual gel and covered again with a moist primary dressing (without REGRANEX gel) for the remainder of the day. REGRANEX should be applied once daily to the ulcer until complete healing has occurred. If the ulcer does not decrease in size by approximately 30% after 10 weeks of treatment or complete healing has not occurred in 20 weeks, continued treatment with REGRANEX should be reassessed. The step-by-step instructions for applying REGRANEX in a home care setting can be found in the FDA-approved Instructions for Use.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Cancer
REGRANEX contains becaplermin, a recombinant human platelet-derived growth factor, which promotes cellular proliferation and angiogenesis [see Clinical Pharmacology (12.1)]. Malignancies distant from the site of application have occurred in REGRANEX users in a clinical study and in postmarketing use [see Adverse Reactions (6.1)andClinical Studies (14.3)].
The benefits and risks of REGRANEX treatment should be carefully evaluated before prescribing in patients with known malignancy.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- In clinical trials, erythematous rashes occurred in 2% of subjects treated with REGRANEX (and good ulcer care) or placebo (and good ulcer care), and none in subjects receiving good ulcer care alone. Subjects treated with REGRANEX did not develop neutralizing antibodies against becaplermin.
- In a retrospective follow-up study of 491 of 651 subjects (75%) from two randomized, controlled trials of another formulation of becaplermin gel 0.01%, the subjects were followed for a median of approximately 20 months to evaluate safety and recurrence of healed diabetic lower extremity ulcers. Eight of 291 subjects (2.7%) from the becaplermin gel group and two of 200 subjects (1%) from the vehicle/standard of care group were diagnosed with cancers during the follow-up period, a relative risk of 2.7 (95% confidence interval [CI], 0.6-12.8). The types of cancers varied and all were remote from the treatment site [see Warnings and Precautions (5.1)].
6.2 Postmarketing Experience
Because post-approval adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to the drug. The following adverse reactions have been identified during post approval use of REGRANEX.
- Increased rate of death from systemic malignancies in patients dispensed 3 or more tubes of REGRANEX was observed in one of three retrospective postmarketing studies [see Clinical Studies (14.3)].
- Burning sensation and erythema at the site of application have been reported.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on REGRANEX use in pregnant women to inform a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Animal reproduction studies have not been conducted with REGRANEX.
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
There are no data on the presence of becaplermin in human milk, the effects on the breastfed infant, or the effects on milk production after topical application of REGRANEX to lactating women. The developmental and health benefits of breastfeeding should be considered along with the lactating woman's clinical need for REGRANEX and any potential adverse effects on the breastfed child from becaplermin.
8.4 Pediatric Use
Safety and effectiveness of REGRANEX in pediatric patients below the age of 16 years have not been established.
8.5 Geriatric Use
Among subjects receiving any dose of REGRANEX in clinical studies of diabetic lower extremity ulcers, 150 subjects were 65 years of age and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. The number of subjects aged 75 and older was insufficient (n=34) to determine whether they respond differently from younger subjects.
- 10 OVERDOSAGE
-
11 DESCRIPTION
REGRANEX contains becaplermin, a recombinant human platelet-derived growth factor, for topical administration. Becaplermin is produced by recombinant DNA technology by insertion of the gene for the B chain of platelet-derived growth factor (PDGF) into the yeast, Saccharomyces cerevisiae.
Becaplermin has a molecular weight of approximately 25 kD and is a homodimer composed of two identical polypeptide chains that are bound together by disulfide bonds. REGRANEX is a non-sterile, low bioburden, preserved, sodium carboxymethylcellulose-based (CMC) topical gel, containing the active ingredient becaplermin and the following inactive ingredients: carboxymethylcellulose sodium, acetic acid, lysine hydrochloride, metacresol, methylparaben, propylparaben, sodium acetate, sodium chloride and purified water. Each gram of REGRANEX contains 100 mcg of becaplermin.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
REGRANEX has biological activity similar to that of endogenous platelet-derived growth factor, which includes promoting the chemotactic recruitment and proliferation of cells involved in wound repair and enhancing the formation of granulation tissue.
12.3 Pharmacokinetics
Ten subjects with Stage III or IV [as defined in the International Association of Enterostomal Therapy (IAET) guide to chronic wound staging] lower extremity diabetic ulcers received topical applications of becaplermin gel 0.01% at a dose range of 0.32–2.95 mcg/kg (7 mcg/cm 2) daily for 14 days. Six subjects had non-quantifiable PDGF levels at baseline and throughout the study, two subjects had PDGF levels at baseline which did not increase substantially, and two subjects had PDGF levels that increased sporadically above their baseline values during the 14-day study period.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Becaplermin was not genotoxic in a battery of in vitroassays (including those for bacterial and mammalian cell point mutation, chromosomal aberration, and DNA damage/repair). Becaplermin was also not mutagenic in an in vivoassay for the induction of micronuclei in mouse bone marrow cells.
Carcinogenesis and reproductive toxicity studies have not been conducted with REGRANEX.
13.2 Animal Toxicology and/or Pharmacology
In nonclinical studies, rats injected at the metatarsals with 3 or 10 mcg/site (approximately 60 or 200 mcg/kg) of becaplermin every other day for 13 days displayed histological changes indicative of accelerated bone remodeling consisting of periosteal hyperplasia and subperiosteal bone resorption and exostosis. The soft tissue adjacent to the injection site had fibroplasia with accompanying mononuclear cell infiltration reflective of the ability of PDGF to stimulate connective tissue growth.
-
14 CLINICAL STUDIES
14.1 Efficacy in Diabetic Lower Extremity Ulcers
The effects of REGRANEX on the incidence of and time to complete healing in lower extremity diabetic neuropathic ulcers were assessed in four randomized controlled studies (Studies 1-4). Of 922 subjects studied, 478 received either REGRANEX 0.003% or 0.01%. All study participants had lower extremity diabetic neuropathic ulcers that extended into the subcutaneous tissue or beyond [Stages III and IV of the International Association of Enterostomal Therapy (IAET) guide to chronic wound staging]. Ninety-three percent of the subjects enrolled in these four trials had foot ulcers. The remaining 7% of the subjects had ankle or leg ulcers. The diabetic ulcers were of at least 8 weeks duration and had an adequate blood supply (defined as T cpO 2> 30 mm Hg). In the four trials, 95% of the ulcers measured in area up to 10 cm 2, and the median ulcer size at baseline ranged from 1.4 cm 2to 3.5 cm 2.
All treatment groups received a program of good ulcer care consisting of initial complete sharp debridement, a non-weight-bearing regimen, systemic treatment for wound-related infection if present, moist saline dressings changed twice a day, and additional debridement as necessary. REGRANEX 0.003% or 0.01% or placebo was applied once a day and covered with a saline moistened dressing. After approximately 12 hours, the gel was gently rinsed off and a saline moistened dressing was then applied for the remainder of the day. Subjects were treated until complete healing, or for a period of up to 20 weeks. Subjects were considered a treatment failure if their ulcer did not show an approximately 30% reduction in initial ulcer area after eight to ten weeks of therapy.
Results of the primary endpoints from 4 independent studies, shown as incidence of complete ulcer closure within 20 weeks, for all treatment arms are given in Figure 1. In each study, REGRANEX in conjunction with good ulcer care was compared to placebo gel plus good ulcer care or good ulcer care alone.
In Study 1, a multicenter, double-blind, placebo-controlled trial of 118 subjects, the incidence of complete ulcer closure for REGRANEX 0.003% (n=61) was 48% versus 25% for placebo gel (n=57; p=0.02, logistic regression analysis).
In Study 2, a multicenter, double-blind, placebo-controlled trial of 382 subjects, the incidence of complete ulcer closure for REGRANEX 0.01% (n=123) was 50% versus 36% for REGRANEX 0.003% (n=132) and 35% for placebo gel (n=127). Only REGRANEX 0.01% was significantly different from placebo gel (p=0.01, logistic regression analysis).
The primary goal of Study 3, a multicenter controlled trial of 172 subjects, was to assess the safety of vehicle gel (placebo; n=70) compared to good ulcer care alone (n=68). The study included a small (n=34) REGRANEX 0.01% arm. Incidences of complete ulcer closure were 44% for REGRANEX, 36% for placebo gel and 22% for good ulcer care alone.
In Study 4, a multicenter, evaluator-blind, controlled trial of 250 subjects, the incidences of complete ulcer closure in the REGRANEX 0.01% arm (n=128) (36%) and good ulcer care alone (n=122) (32%) were not statistically different.
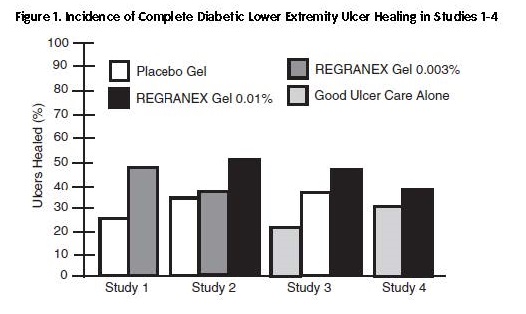
In general, where REGRANEX was associated with higher incidences of complete ulcer closure, differences in the incidence first became apparent after approximately 10 weeks and increased with continued treatment (Table 3).
Table 3: Life Table Estimates of the Incidence (%) of Complete Diabetic Lower Extremity Ulcer Healing over Time of Study 2
REGRANEX
Gel 0.01%
(%)
Placebo
(%)
Week 2
1
0
Week 4
6
2
Week 6
9
6
Week 8
16
14
Week 10
23
18
Week 12
34
25
Week 14
37
28
Week 16
43
33
Week 18
46
34
Week 20
50
37
In a 3-month follow-up period where no standardized regimen of preventative care was utilized, the incidence of ulcer recurrence was approximately 30% in all treatment groups, demonstrating that the durability of ulcer closure was comparable in all treatment groups.
14.2 Lack of Efficacy in Pressure Ulcers and Venous Stasis Ulcers
In a randomized, double-blind study of REGRANEX (100 mcg/g once daily for 16 weeks) in subjects with Stage III or IV pressure ulcers, the incidence of complete ulcer closure was 15% (28/189) in the REGRANEX group and 12% (22/190) in the vehicle control group. This difference was not statistically significant.
In two small, randomized, double-blinded studies of REGRANEX (100 mcg/g once daily for 16 weeks) in subjects with venous stasis ulcers, the combined incidence of complete ulcer closure was 46% (30/65) in the REGRANEX group and 39% (26/67) in the vehicle control group. This difference was not statistically significant.
14.3 Observational Studies to Evaluate Cancer Development and Mortality
The observational studies described below do not involve random allocation of treatments. They are susceptible to bias and confounding.
A retrospective study using medical claims database to assess cancer incidence with up to 6 years of follow-up observed development of 28 cancers and 8 cancer deaths in the REGRANEX exposed cohort (n = 1,622) and 43 cancers and 8 cancer deaths in the matched comparator cohort not exposed to REGRANEX (n = 2,809). The rate ratio for incident cancer comparing the REGRANEX-exposed cohort to the unexposed comparator cohort was 1.2 (95% CI, 0.7 -1.9). The rate ratio for cancer mortality comparing the REGRANEX-exposed cohort to the unexposed comparator cohort was 1.8 (95% CI, 0.7 -4.9). The rate ratio comparing patients exposed to three or more tubes of REGRANEX to those not exposed was 5.2 (95% CI, 1.6 -17.6) [see Adverse Reactions (6.2)].
A retrospective study using medical claims from the Veteran Affairs health care database with up to 11 years of follow-up among patients withoutprior cancer observed 197 cancer deaths in the REGRANEX exposed cohort (n = 6,429) and 206 cancer deaths in the matched comparator cohort not exposed to REGRANEX (n = 6,429), resulting in a hazard ratio of 0.9 (95% CI, 0.8-1.2). The hazard ratio for cancer mortality comparing patients exposed to three or more tubes of REGRANEX to those not exposed was 1.0 (95% CI, 0.7 -1.5). The hazard ratio for incident cancer in a smaller cohort (1,507 REGRANEX-exposed and 1,507 unexposed patients) comparing patients exposed to REGRANEX to those not exposed was 1.1 (95% CI, 0.8-1.4).
A second retrospective study using medical claims from the Veteran Affairs health care database with up to 11 years of follow-up among patients withprior cancer observed 87 cancer deaths in the REGRANEX-exposed cohort (n = 477) and 340 cancer deaths in the matched comparator cohort not exposed to REGRANEX (n = 1,756), resulting in a hazard ratio of 0.9 (95% CI, 0.7-1.2). The hazard ratio for cancer mortality comparing patients exposed to three or more tubes of REGRANEX to those not exposed was 0.9 (95% CI, 0.6 -1.2).
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
- Advise patients and caregivers to read the FDA-approved patient labeling (Medication Guide and Instructions for Use) and follow the step-by-step instructions for REGRANEX application in the Instructions for Use.
- Counsel patients to review and discuss any questions or concerns with their healthcare provider before starting REGRANEX and at regular intervals while receiving REGRANEX.
- Advise patients that it is important to use REGRANEX together with a good ulcer care program, including a strict non-weight-bearing program.
- Advise patients to store REGRANEX in the refrigerator and not to freeze REGRANEX.
Manufactured and marketed by: Smith & Nephew, Inc., Fort Worth, TX 76109
U.S. Gov’t License # 2004
REGRANEX ®is a registered trademark of Smith & Nephew, Inc.
Part No. 141125-0819
-
MEDICATION GUIDE
MEDICATION GUIDE
REGRANEX®(RE–GRANʹ–IX)
(becaplermin)
gel
Important:REGRANEX is for use on the skin only (topical). Do not use REGRANEX near or in your mouth, eyes, or vagina.
What is the most important information I should know about REGRANEX?
REGRANEX may cause serious side effects, including:
Risk of cancer.Cancers have happened in areas away from the REGRANEX application site. You and your healthcare provider should carefully consider whether you will use REGRANEX if you have cancer.
What is REGRANEX?
REGRANEX is a prescription medicine that is used with good ulcer care practice for the treatment of diabetic sores (ulcers) of your legs or feet that are deeper than just your skin, in people who have good blood supply to the legs and feet.
It is not known if REGRANEX is effective for the treatment of pressure ulcers or ulcers that are due to poor blood flow (circulation).
It is not known if REGRANEX is safe and effective in children under 16 years of age.
Who should not use REGRANEX?
Do not use REGRANEXif you have a cancer at the application site.
Before using REGRANEX, tell your healthcare provider about all of your medical conditions, including if you:
- have cancer.
- are pregnant or plan to become pregnant. It is not known if REGRANEX will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if REGRANEX passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with REGRANEX.
Tell your healthcare provider about all the medicines you take,including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use REGRANEX?
Read the Instructions for Use for detailed information about the right way to apply REGRANEX.
- Use REGRANEX together with good ulcer care, as prescribed by your healthcare provider. This includes following your healthcare provider's instructions about not putting weight (non-weight bearing) on the affected leg and foot.
- Use REGRANEX exactly as your healthcare provider tells you to use it.
- The amount of REGRANEX you will apply will depend on the size of your ulcer. Your healthcare provider should check the size of your ulcer every 1 to 2 weeks. Your healthcare provider may change the amount of REGRANEX to be applied to your ulcer as the size of your ulcer changes.
What are the possible side effects of REGRANEX?
REGRANEX may cause serious side effects.
- See "What is the most important information I should know about REGRANEX?"
- Application site reactions.Tell your healthcare provider if you have any skin reactions such as burning sensation at the site of application during treatment with REGRANEX. Your healthcare provider may temporarily stop or completely stop treatment with REGRANEX if you have skin reactions.
The most common side effect of REGRANEXis red skin rashes.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Smith & Nephew, Inc. at 1-800-441-8227.
How should I store REGRANEX?
- Store REGRANEX in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Do not freeze REGRANEX.
- Do not use REGRANEX after the expiration date on the bottom (sealed end) of the tube.
- Throw away your REGRANEX that is out of date or no longer needed for your treatment.
Keep REGRANEX and all medicines out of the reach of children.
General information about the safe and effective use of REGRANEX
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use REGRANEX for a condition for which it was not prescribed. Do not give REGRANEX to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about REGRANEX that is written for health professionals.
What are the ingredients in REGRANEX?
Active ingredient:becaplermin
Inactive ingredients:carboxymethylcellulose sodium, acetic acid, lysine hydrochloride, metacresol, methylparaben, propylparaben, sodium acetate, sodium chloride and purified water.
Manufactured and marketed by: Smith & Nephew, Inc., Fort Worth, TX 76109
U.S. Gov’t License # 2004
REGRANEX ®is a registered trademark of Smith & Nephew, Inc.
Part No. 141125-0819
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 08/2019
-
SPL UNCLASSIFIED SECTION
INSTRUCTIONS FOR USE
REGRANEX ®(RE-GRAN-IX)
(becaplermin)
gel
Important:REGRANEX is for use on the skin only (topical). Do not use REGRANEX near or in your mouth, eyes, or vagina.
Read these Instructions for Use before you start using REGRANEXand each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. Follow your healthcare provider’s instructions for measuring and applying REGRANEX.
- Apply REGRANEX one time a day to ulcer area.
- Do notuse more than your prescribed dose of REGRANEX or apply more often than every 24 hours.
- Do notlet the tip of your REGRANEX tube touch the ulcer or any other surface.
- Put the REGRANEX tube in the refrigerator after each use.
Supplies you need to apply REGRANEX:
- Clean cotton swab, tongue depressor or similar application aid
- Ruler or measuring tape
- Clean firm, non-absorbable surface, such as wax paper
- Saline moistened gauze dressing
Step 1. Preparing the REGRANEX dose.
- Wash your hands well before you apply REGRANEX.
- Remove the cap from the REGRANEX tube and use the top of the cap to pierce the foil seal on the top of the tube by pushing or screwing the cap in.
- Carefully measure the amount of REGRANEX that your healthcare provider tells you to use.
- Squeeze the amount of REGRANEX needed for your ulcer on to the clean, firm non-absorbable surface and measure to the correct length with a ruler as prescribed by your healthcare provider.
- Close the REGRANEX tube tightly.
Step 2. Applying REGRANEX.
- Use a clean cotton swab, tongue depressor, or similar application aid to spread the measured amount of REGRANEX in a thin even layer over the ulcer area.
- Cover the application site with a saline moistened gauze dressing.
- Wash your hands well.
Step 3. Removing REGRANEX.
- Remove REGRANEX after 12 hours.
- Wash your hands well.
- Remove the saline moistened gauze dressing.
- Rinse the ulcer with saline or water to remove any REGRANEX gel.
- Cover the ulcer with a new moistened dressing.
- Wash your hands well.
How should I store REGRANEX?
- Store REGRANEX in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Do not freeze REGRANEX.
- Do not use REGRANEX after the expiration date on the bottom (sealed end) of the tube.
- Throw away your REGRANEX that is out of date or no longer needed for your treatment.
Keep REGRANEX and all medicines out of the reach of children.
This "Instructions for Use" has been approved by the U.S. Food and Drug Administration. Issued: 08/2019
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
REGRANEX
becaplermin gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50484-810 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BECAPLERMIN (UNII: 1B56C968OA) (BECAPLERMIN - UNII:1B56C968OA) BECAPLERMIN 100 ug in 1 g Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) ACETIC ACID (UNII: Q40Q9N063P) LYSINE HYDROCHLORIDE (UNII: JNJ23Q2COM) METACRESOL (UNII: GGO4Y809LO) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM ACETATE (UNII: 4550K0SC9B) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Product Characteristics Color yellow (CLEAR, COLORLESS TO STRAW-COLORED) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50484-810-15 1 in 1 CARTON 08/01/2014 1 15 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103691 11/01/2011 Labeler - Smith & Nephew, Inc. (827731451) Registrant - Smith & Nephew, Inc. (827731451) Establishment Name Address ID/FEI Business Operations Smith & Nephew Wound Management 125458849 manufacture(50484-810)




