Label: lupron- leuprolide acetate
lupron- leuprolide acetate injection, solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 0300-3612-24, 0300-3612-28 - Packager: TAP Pharmaceutical Products Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 29, 2008
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- N/A - Section Title Not Found In Database
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
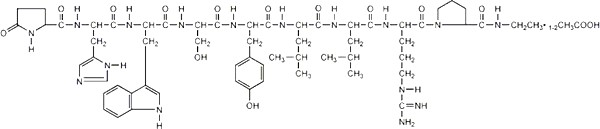
Leuprolide acetate is a synthetic nonapeptide analog of naturally occurring gonadotropin releasing hormone (GnRH or LH-RH). The analog possesses greater potency than the natural hormone. The chemical name is 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-D-leucyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide acetate (salt) with the following structural formula:
LUPRON INJECTION is a sterile, aqueous solution intended for subcutaneous injection. It is available in a 2.8 mL multiple-dose vial containing leuprolide acetate (5 mg/mL), sodium chloride, USP (6.3 mg/mL) for tonicity adjustment, benzyl alcohol, NF as a preservative (9 mg/mL), and water for injection, USP. The pH may have been adjusted with sodium hydroxide, NF and/or acetic acid, NF.
-
CLINICAL PHARMACOLOGY
Leuprolide acetate, an LH-RH agonist, acts as a potent inhibitor of gonadotropin secretion when given continuously and in therapeutic doses. Animal and human studies indicate that following an initial stimulation of gonadotropins, chronic administration of leuprolide acetate results in suppression of ovarian and testicular steroidogenesis. This effect is reversible upon discontinuation of drug therapy. Administration of leuprolide acetate has resulted in inhibition of the growth of certain hormone dependent tumors (prostatic tumors in Noble and Dunning male rats and DMBA-induced mammary tumors in female rats) as well as atrophy of the reproductive organs.
In humans, subcutaneous administration of single daily doses of leuprolide acetate results in an initial increase in circulating levels of luteinizing hormone (LH) and follicle stimulating hormone (FSH), leading to a transient increase in levels of the gonadal steroids (testosterone and dihydrotestosterone in males, and estrone and estradiol in pre-menopausal females). However, continuous daily administration of leuprolide acetate results in decreased levels of LH and FSH. In males, testosterone is reduced to castrate levels. In pre-menopausal females, estrogens are reduced to post-menopausal levels. These decreases occur within two to four weeks after initiation of treatment, and castrate levels of testosterone in prostatic cancer patients have been demonstrated for periods of up to five years.
Leuprolide acetate is not active when given orally.
Pharmacokinetics
Absorption
Bioavailability by subcutaneous administration is comparable to that by intravenous administration.
Distribution
The mean steady-state volume of distribution of leuprolide following intravenous bolus administration to healthy male volunteers was 27 L. In vitro binding to human plasma proteins ranged from 43% to 49%.
Metabolism
In healthy male volunteers, a 1 mg bolus of leuprolide administered intravenously revealed that the mean systemic clearance was 7.6 L/h, with a terminal elimination half-life of approximately 3 hours based on a two compartment model. In rats and dogs, administration of 14C-labeled leuprolide was shown to be metabolized to smaller inactive peptides, a pentapeptide (Metabolite I), tripeptides (Metabolites II and III) and a dipeptide (Metabolite IV). These fragments may be further catabolized.
The major metabolite (M-I) plasma concentrations measured in 5 prostate cancer patients reached maximum concentration 2 to 6 hours after dosing and were approximately 6% of the peak parent drug concentration. One week after dosing, mean plasma M-I concentrations were approximately 20% of mean leuprolide concentrations.
Excretion
Following administration of LUPRON DEPOT 3.75 mg to 3 patients, less than 5% of the dose was recovered as parent and M-I metabolite in the urine.
Special Populations
The pharmacokinetics of the drug in hepatically and renally impaired patients has not been determined.
Drug Interactions
No pharmacokinetic-based drug-drug interaction studies have been conducted with leuprolide acetate. However, because leuprolide acetate is a peptide that is primarily degraded by peptidase and not by cytochrome P-450 enzymes as noted in specific studies, and the drug is only about 46% bound to plasma proteins, drug interactions would not be expected to occur.
- CLINICAL STUDIES
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
- LUPRON INJECTION is contraindicated in patients known to be hypersensitive to GnRH, GnRH agonist analogs or any of the excipients in LUPRON INJECTION: Reports of anaphylactic reactions to GnRH agonist analogs have been reported in the medical literature.1,2
- LUPRON is contraindicated in women who are or may become pregnant while receiving the drug. LUPRON may cause fetal harm when administered to a pregnant woman. Therefore, the possibility exists that spontaneous abortion may occur if the drug is administered during pregnancy. If this drug is administered during pregnancy or if the patient becomes pregnant while taking any formulation of LUPRON, the patient should be apprised of the potential hazard to the fetus.
-
WARNINGS
Initially, LUPRON, like other LH-RH agonists, causes increases in serum levels of testosterone. Transient worsening of symptoms, or the occurrence of additional signs and symptoms of prostate cancer, may occasionally develop during the first few weeks of LUPRON treatment. A small number of patients may experience a temporary increase in bone pain, which can be managed symptomatically. As with other LH-RH agonists, isolated cases of ureteral obstruction and spinal cord compression have been observed, which may contribute to paralysis with or without fatal complications.
Safe use of leuprolide acetate in pregnancy has not been established clinically. Before starting treatment with LUPRON, pregnancy must be excluded (see CONTRAINDICATIONS section).
Periodic monitoring of serum testosterone and prostate-specific antigen (PSA) levels is recommended, especially if the anticipated clinical or biochemical response to treatment has not been achieved. It should be noted that results of testosterone determinations are dependent on assay methodology. It is advisable to be aware of the type and precision of the assay methodology to make appropriate clinical and therapeutic decisions.
-
PRECAUTIONS
Patients with metastatic vertebral lesions and/or with urinary tract obstruction should be closely observed during the first few weeks of therapy (see WARNINGS and ADVERSE REACTIONS sections).
Patients with known allergies to benzyl alcohol, an ingredient of the drug's vehicle, may present symptoms of hypersensitivity, usually local, in the form of erythema and induration at the injection site.
Laboratory Tests
Response to leuprolide acetate should be monitored by measuring serum levels of testosterone and prostate-specific antigen (PSA). In the majority of patients, testosterone levels increased above baseline during the first week, declining thereafter to baseline levels or below by the end of the second week of treatment. Castrate levels were reached within two to four weeks and once attained were maintained for as long as drug administration continued.
Drug/Laboratory Test Interactions
Administration of leuprolide acetate in therapeutic doses results in suppression of the pituitary-gonadal system. Normal function is usually restored within 4 to 12 weeks after treatment is discontinued.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies were conducted in rats and mice. In rats, a dose-related increase of benign pituitary hyperplasia and benign pituitary adenomas was noted at 24 months when the drug was administered subcutaneously at high daily doses (0.6 to 4 mg/kg). There was a significant but not dose-related increase of pancreatic islet-cell adenomas in females and of testicular interstitial cell adenomas in males (highest incidence in the low dose group). In mice no pituitary abnormalities were observed at a dose as high as 60 mg/kg for two years. Patients have been treated with leuprolide acetate for up to three years with doses as high as 10 mg/day and for two years with doses as high as 20 mg/day without demonstrable pituitary abnormalities.
Mutagenicity studies have been performed with leuprolide acetate using bacterial and mammalian systems. These studies provided no evidence of a mutagenic potential.
Clinical and pharmacologic studies in adults (≥ 18 years) with leuprolide acetate and similar analogs have shown full reversibility of fertility suppression when the drug is discontinued after continuous administration for periods of up to 24 weeks. However, no clinical studies have been conducted with leuprolide acetate to assess the reversibility of fertility suppression.
Pregnancy
Teratogenic Effects
Pregnancy Category X
(see CONTRAINDICATIONS and WARNINGS sections)
When administered on day 6 of pregnancy at test dosages of 0.00024, 0.0024, and 0.024 mg/kg (1/600 to 1/6 the human dose) to rabbits, LUPRON produced a dose-related increase in major fetal abnormalities. Similar studies in rats failed to demonstrate an increase in major fetal malformations throughout gestation. There was increased fetal mortality and decreased fetal weights with the two higher doses of LUPRON in rabbits and with the highest dose in rats. The effects on fetal mortality are expected consequences of the alterations in hormonal levels brought about by this drug.
Nursing Mothers
It is not known whether leuprolide acetate is excreted in human milk. LUPRON should not be used by nursing mothers.
Pediatric Use
See labeling for LUPRON INJECTION for Pediatric Use for the safety and effectiveness in children with central precocious puberty.
-
ADVERSE REACTIONS
Clinical Trials
In the majority of patients testosterone levels increased above baseline during the first week, declining thereafter to baseline levels or below by the end of the second week of treatment. This transient increase was occasionally associated with a temporary worsening of signs and symptoms, usually manifested by an increase in bone pain (see WARNINGS section). In a few cases a temporary worsening of existing hematuria and urinary tract obstruction occurred during the first week. Temporary weakness and paresthesia of the lower limbs have been reported in a few cases.
Potential exacerbations of signs and symptoms during the first few weeks of treatment is a concern in patients with vertebral metastases and/or urinary obstruction which, if aggravated, may lead to neurological problems or increase the obstruction.
In a comparative trial of LUPRON INJECTION (leuprolide acetate) versus DES, in 5% or more of the patients receiving either drug, the following adverse reactions were reported to have a possible or probable relationship to drug as ascribed by the treating physician. Often, causality is difficult to assess in patients with metastatic prostate cancer. Reactions considered not drug related are excluded.
LUPRON
(N=98)DES
(N=101)Number of Reports - *
- Physiologic effect of decreased testosterone.
Cardiovascular System Congestive heart failure 1 5 ECG changes/ischemia 19 22 High blood pressure 8 5 Murmur 3 8 Peripheral edema 12 30 Phlebitis/thrombosis 2 10 Gastrointestinal System Anorexia 6 5 Constipation 7 9 Nausea/vomiting 5 17 Endocrine System *Decreased testicular size 7 11 *Gynecomastia/breast tenderness or pain 7 63 *Hot flashes 55 12 *Impotence 4 12 Hemic and Lymphatic System Anemia 5 5 Musculoskeletal System Bone pain 5 2 Myalgia 3 9 Central/Peripheral Nervous System Dizziness/lightheadedness 5 7 General pain 13 13 Headache 7 4 Insomnia/sleep disorders 7 5 Respiratory System Dyspnea 2 8 Sinus congestion 5 6 Integumentary System Dermatitis 5 8 Urogenital System Frequency/urgency 6 8 Hematuria 6 4 Urinary tract infection 3 7 Miscellaneous Asthenia 10 10 In this same study, the following adverse reactions were reported in less than 5% of the patients on LUPRON.
Cardiovascular System—Angina, Cardiac arrhythmias, Myocardial infarction, Pulmonary emboli; Gastrointestinal System—Diarrhea, Dysphagia, Gastrointestinal bleeding, Gastrointestinal disturbance, Peptic ulcer, Rectal polyps; Endocrine System—Libido decrease, Thyroid enlargement; Musculoskeletal System—Joint pain; Central/Peripheral Nervous System—Anxiety, Blurred vision, Lethargy, Memory disorder, Mood swings, Nervousness, Numbness, Paresthesia, Peripheral neuropathy, Syncope/blackouts, Taste disorders; Respiratory System—Cough, Pleural rub, Pneumonia, Pulmonary fibrosis; Integumentary System—Carcinoma of skin/ear, Dry skin, Ecchymosis, Hair loss, Itching, Local skin reactions, Pigmentation, Skin lesions; Urogenital System—Bladder spasms, Dysuria, Incontinence, Testicular pain, Urinary obstruction; Miscellaneous—Depression, Diabetes, Fatigue, Fever/chills, Hypoglycemia, Increased BUN, Increased calcium, Increased creatinine, Infection/inflammation, Ophthalmologic disorders, Swelling (temporal bone).
In an additional clinical trial and from long-term observation of both studies, the following additional adverse events (excluding those considered not drug related) were reported for patients receiving LUPRON.
Cardiovascular System—Bradycardia, Carotid bruit, Extrasystole, Palpitations, Perivascular cuffing (eyes), Ruptured aortic aneurysm, Stroke, Tachycardia, Transient ischemic attack; Gastrointestinal System—Flatus, Dryness of mouth and throat, Hepatitis, Hepatomegaly, Occult blood (rectal exam), Rectal fistula/erythema; Endocrine System—Libido increase, Thyroid nodule; Musculoskeletal System—Ankylosing spondylosis, Arthritis, Blurred disc margins, Bone fracture, Muscle stiffness, Muscle tenderness, Pelvic fibrosis, Spasms/cramps; Central/Peripheral Nervous System—Auditory hallucinations/tinnitus, Decreased hearing, Decreased reflexes, Euphoria, Hyperreflexia, Loss of smell, Motor deficiency; Respiratory System—Chest tightness, Decreased breathing sounds, Hemoptysis, Pleuritic chest pain, Pulmonary infiltrate, Rales/rhonchi, Rhinitis, Strep throat, Wheezing/bronchitis; Integumentary System—Boil (pubic), Bruises, Hives, Keratosis, Mole, Shingles, Spiders; Urogenital System— Blisters on penis, Inguinal hernia, Penile swelling, Post void residual, Prostatic pain, Pyuria; Miscellaneous—Abdominal distention, Facial swelling/edema, Feet burning, Flu, Eyelid growth, Hypoproteinemia, Accidental injury, Knee effusion, Mass, Pallid, Sallow, Weakness.
Postmarketing
During postmarketing surveillance which includes other dosage forms and other patient populations, the following adverse events were reported.
Symptoms consistent with an anaphylactoid or asthmatic process have been rarely (incidence rate of about 0.002%) reported. Rash, urticaria, and photosensitivity reactions have also been reported.
Localized reactions including induration and abscess have been reported at the site of injection.
Symptoms consistent with fibromyalgia (e.g., joint and muscle pain, headaches, sleep disorders, gastrointestinal distress, and shortness of breath) have been reported individually and collectively.
Cardiovascular System – Hypotension, Myocardial infarction; Endocrine System - Diabetes; Gastrointestinal System – Hepatic dysfunction; Hemic and Lymphatic System – Decreased WBC; Integumentary System – Hair growth; Central/Peripheral Nervous System – Spinal fracture/paralysis, Hearing disorder; Miscellaneous – Hard nodule in throat, Weight gain, Increased uric acid; Musculoskeletal System – Tenosynovitis-like symptoms; Respiratory System – Respiratory disorders.
Changes in Bone Density: Decreased bone density has been reported in the medical literature in men who have had orchiectomy or who have been treated with an LH-RH agonist analog. In a clinical trial, 25 men with prostate cancer, 12 of whom had been treated previously with leuprolide acetate for at least six months, underwent bone density studies as a result of pain. The leuprolide-treated group had lower bone density scores than the nontreated control group. It can be anticipated that long periods of medical castration in men will have effects on bone density.
Pituitary apoplexy: During post-marketing surveillance, rare cases of pituitary apoplexy (a clinical syndrome secondary to infarction of the pituitary gland) have been reported after the administration of gonadotropin-releasing hormone agonists. In a majority of these cases, a pituitary adenoma was diagnosed, with a majority of pituitary apoplexy cases occurring within 2 weeks of the first dose, and some within the first hour. In these cases, pituitary apoplexy has presented as sudden headache, vomiting, visual changes, ophthalmoplegia, altered mental status, and sometimes cardiovascular collapse. Immediate medical attention has been required.
See other LUPRON DEPOT and LUPRON INJECTION package inserts for other events reported in the same and different patient populations.
-
OVERDOSAGE
In rats subcutaneous administration of 250 to 500 times the recommended human dose, expressed on a per body weight basis, resulted in dyspnea, decreased activity, and local irritation at the injection site. There is no evidence at present that there is a clinical counterpart of this phenomenon. In early clinical trials with leuprolide acetate doses as high as 20 mg/day for up to two years caused no adverse effects differing from those observed with the 1 mg/day dose.
-
DOSAGE AND ADMINISTRATION
The recommended dose is 1 mg (0.2 mL or 20 unit mark) administered as a single daily subcutaneous injection. As with other drugs administered chronically by subcutaneous injection, the injection site should be varied periodically. Each 0.2 mL contains 1 mg of leuprolide acetate, sodium chloride for tonicity adjustment, 1.8 mg of benzyl alcohol as preservative and water for injection. The pH may have been adjusted with sodium hydroxide and/or acetic acid.
Follow the pictorial directions on the reverse side of this package insert for administration.
NOTE: As with all parenteral products, inspect the solution for discoloration and particulate matter before each use.
-
HOW SUPPLIED
LUPRON INJECTION (leuprolide acetate) is a sterile solution supplied in a 2.8 mL multiple-dose vial. The vial is packaged as follows: 14 Day Patient Administration Kit with 14 disposable syringes and 28 alcohol swabs, NDC 0300-3612-28 and six-vial carton, NDC 0300-3612-24.
Store below 77°F (25°C). Do not freeze. Protect from light; store vial in carton until use.
U.S. Patent Nos. 4,005,063 and 4,005,194.
- REFERENCES
-
SUPPLEMENTAL PATIENT MATERIAL
INFORMATION FOR PATIENTS
Be sure to consult your physician with any questions you may have or for information about LUPRON INJECTION (leuprolide acetate) and its use.
WHAT IS LUPRON?
LUPRON INJECTION (leuprolide acetate) is chemically similar to gonadotropin releasing hormone (GnRH or LH-RH) a hormone which occurs naturally in your body.
Normally, your body releases small amounts of LH-RH and this leads to events which stimulate the production of sex hormones.
However, when you inject LUPRON INJECTION (leuprolide acetate), the normal events that lead to sex hormone production are interrupted and testosterone is no longer produced by the testes.
LUPRON must be injected because, like insulin which is injected by diabetics, LUPRON is inactive when taken by mouth.
If you were to discontinue the drug for any reason, your body would begin making testosterone again.
DIRECTIONS FOR USING LUPRON
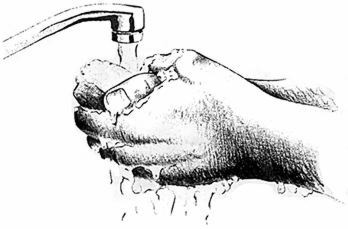
- Wash hands thoroughly with soap and water.
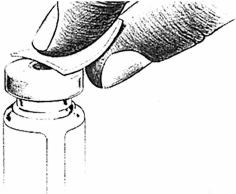
- If using a new bottle for the first time, flip off the plastic cover to expose the grey rubber stopper. Wipe metal ring and rubber stopper with an alcohol wipe each time you use LUPRON. Check the liquid in the container. If it is not clear or has particles in it, DO NOT USE IT. Exchange it at your pharmacy for another container.
- Remove outer wrapping from one syringe. Pull plunger back until the tip of the plunger is at the 0.2 mL or 20 unit mark.
- Take cover off needle. Push the needle through the center of the rubber stopper on the LUPRON bottle.
- Push the plunger all the way in to inject air into the bottle.
- Keep the needle in the bottle and turn the bottle upside down. Check to make sure the tip of the needle is in the liquid. Slowly pull back on the plunger, until the syringe fills to the 0.2 mL or 20 unit mark.
- Toward the end of a two-week period, the amount of LUPRON left in the bottle will be small. Take special care to hold the bottle straight and to keep the needle tip in liquid while pulling back on the plunger.
- Keeping the needle in the bottle and the bottle upside down, check for air bubbles in the syringe. If you see any, push the plunger slowly in to push the air bubble back into the bottle. Keep the tip of the needle in the liquid and pull the plunger back again to fill to the 0.2 mL or 20 unit mark.
- Do this again if necessary to eliminate air bubbles.
- To protect your skin, inject each daily dose at a different body spot.
- Choose an injection spot. Cleanse the injection spot with another alcohol wipe.
- Hold the syringe in one hand. Hold the skin taut, or pull up a little flesh with the other hand, as you were instructed.
- Holding the syringe as you would a pencil, thrust the needle all the way into the skin at a 90° angle. Push the plunger to administer the injection.
- Hold an alcohol wipe down on your skin where the needle is inserted and withdraw the needle at the same angle it was inserted.
- Use the disposable syringe only once and dispose of it properly as you were instructed. Needles thrown into a garbage bag could accidentally stick someone. NEVER LEAVE SYRINGES, NEEDLES OR DRUGS WHERE CHILDREN CAN REACH THEM.
SOME SPECIAL ADVICE
- You may experience hot flashes when using LUPRON INJECTION (leuprolide acetate). During the first few weeks of treatment you may experience increased bone pain, increased difficulty in urinating, and less commonly but most importantly, you may experience the onset or aggravation of nerve symptoms. In any of these events, discuss the symptoms with your doctor. Like other treatment options, LUPRON may cause impotence. Notify your doctor if you develop new or worsened symptoms after beginning LUPRON treatment.
- You may experience some irritation at the injection site, such as burning, itching or swelling. These reactions are usually mild and go away. If they do not, tell your doctor.
- If you have experienced an allergic reaction to other drugs like LUPRON, you should not use this drug.
- Do not stop taking your injections because you feel better. You need an injection every day to make sure LUPRON keeps working for you.
- If you need to use an alternate to the syringe supplied with LUPRON, low-dose insulin syringes should be utilized.
- When the drug level gets low, take special care to hold the bottle straight up and down and to keep the needle tip in liquid while pulling back on the plunger.
- Do not try to get every last drop out of the bottle. This will increase the possibility of drawing air into the syringe and getting an incomplete dose. Some extra drug has been provided so that you can withdraw the recommended number of doses.
- Tell your pharmacist when you will need LUPRON so it will be at the pharmacy when you need it.
- Store below 77°F (25°C). Do not store near a radiator or other very warm place. Do not freeze. Protect from light; store vial in carton until use.
- Do not leave your drug or hypodermic syringes where anyone can pick them up.
- Keep this and all other medications out of reach of children.
- SPL UNCLASSIFIED SECTION
- For Pediatric Use
-
DESCRIPTION
Leuprolide acetate is a synthetic nonapeptide analog of naturally occurring gonadotropin releasing hormone (GnRH or LH-RH). The analog possesses greater potency than the natural hormone. The chemical name is 5- oxo -L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-D-leucyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide acetate (salt) with the following structural formula:

LUPRON INJECTION is a sterile, aqueous solution intended for daily subcutaneous injection.
It is available in a 2.8 mL multiple dose vial containing leuprolide acetate (5 mg/mL), sodium chloride, USP (6.3 mg/mL) for tonicity adjustment, benzyl alcohol, NF as a preservative (9 mg/mL), and water for injection, USP. The pH may have been adjusted with sodium hydroxide, NF and/or acetic acid, NF.
-
CLINICAL PHARMACOLOGY
Leuprolide acetate, a GnRH agonist, acts as a potent inhibitor of gonadotropin secretion when given continuously and in therapeutic doses. Animal and human studies indicate that following an initial stimulation of gonadotropins, chronic administration of leuprolide acetate results in suppression of ovarian and testicular steroidogenesis. This effect is reversible upon discontinuation of drug therapy.
Leuprolide acetate is not active when given orally.
Pharmacokinetics
A pharmacokinetic study of leuprolide acetate in children has not been performed.
Absorption
In adults, bioavailability by subcutaneous administration is comparable to that by intravenous administration.
Distribution
The mean steady-state volume of distribution of leuprolide following intravenous bolus administration to healthy adult male volunteers was 27 L. In vitro binding to human plasma proteins ranged from 43% to 49%.
Metabolism
In healthy adult male volunteers, a 1 mg bolus of leuprolide administered intravenously revealed that the mean systemic clearance was 7.6 L/h, with a terminal elimination half-life of approximately 3 hours based on a two compartment model.
In rats and dogs, administration of 14C-labeled leuprolide was shown to be metabolized to smaller inactive peptides, a pentapeptide (Metabolite I), tripeptides (Metabolites II and III) and a dipeptide (Metabolite IV). These fragments may be further catabolized.
The major metabolite (M-I) plasma concentrations measured in 5 prostate cancer patients reached maximum concentration 2 to 6 hours after dosing and were approximately 6% of the peak parent drug concentration. One week after dosing, mean plasma M-I concentrations were approximately 20% of mean leuprolide concentrations.
Excretion
Following administration of LUPRON DEPOT 3.75 mg to three adult patients, less than 5% of the dose was recovered as parent and M-I metabolite in the urine.
Special Populations
The pharmacokinetics of the drug in hepatically and renally impaired patients has not been determined.
Drug Interactions
No pharmacokinetic-based drug-drug interaction studies have been conducted with leuprolide acetate. However, because leuprolide acetate is a peptide that is primarily degraded by peptidase and the drug is only about 46% bound to plasma proteins, drug interactions would not be expected to occur.
-
CLINICAL STUDIES
In children with central precocious puberty (CPP), stimulated and basal gonadotropins are reduced to prepubertal levels. Testosterone and estradiol are reduced to prepubertal levels in males and females respectively. Reduction of gonadotropins will allow for normal physical and psychological growth and development. Natural maturation occurs when gonadotropins return to pubertal levels following discontinuation of leuprolide acetate.
The following physiologic effects have been noted with the chronic administration of leuprolide acetate in this patient population.
- Skeletal Growth. A measurable increase in body length can be noted since the epiphyseal plates will not close prematurely.
- Organ Growth. Reproductive organs will return to a prepubertal state.
- Menses. Menses, if present, will cease.
-
INDICATIONS AND USAGE
LUPRON INJECTION is indicated in the treatment of children with central precocious puberty. Children should be selected using the following criteria:
- Clinical diagnosis of CPP (idiopathic or neurogenic) with onset of secondary sexual characteristics earlier than 8 years in females and 9 years in males.
- Clinical diagnosis should be confirmed prior to initiation of therapy:
- Confirmation of diagnosis by a pubertal response to a GnRH stimulation test. The sensitivity and methodology of this assay must be understood.
- Bone age advanced 1 year beyond the chronological age.
- Baseline evaluation should also include:
- Height and weight measurements.
- Sex steroid levels.
- Adrenal steroid level to exclude congenital adrenal hyperplasia.
- Beta human chorionic gonadotropin level to rule out a chorionic gonadotropin secreting tumor.
- Pelvic/adrenal/testicular ultrasound to rule out a steroid secreting tumor.
- Computerized tomography of the head to rule out intracranial tumor.
-
CONTRAINDICATIONS
- Hypersensitivity to GnRH, GnRH agonist analogs or any of the excipients in LUPRON INJECTION. Reports of anaphylactic reactions to GnRH agonist analogs have been reported in the medical literature.1,2
- LUPRON is contraindicated in women who are or may become pregnant while receiving the drug. LUPRON may cause fetal harm when administered to a pregnant woman. Major fetal abnormalities were observed in rabbits but not in rats after administration of leuprolide acetate throughout gestation. There was increased fetal mortality and decreased fetal weights in rats and rabbits. (See PRECAUTIONS, Pregnancy, Teratogenic Effects section.) The effects on fetal mortality are expected consequences of the alterations in hormonal levels brought about by this drug. Therefore, the possibility exists that spontaneous abortion may occur if the drug is administered during pregnancy. If this drug is administered during pregnancy or if the patient becomes pregnant while taking any formulation of LUPRON, the patient should be apprised of the potential hazard to the fetus.
-
WARNINGS
During the early phase of therapy, gonadotropins and sex steroids rise above baseline because of the natural stimulatory effect of the drug. Therefore, an increase in clinical signs and symptoms may be observed (see CLINICAL PHARMACOLOGY section).
Noncompliance with drug regimen or inadequate dosing may result in inadequate control of the pubertal process. The consequences of poor control include the return of pubertal signs such as menses, breast development, and testicular growth. The long-term consequences of inadequate control of gonadal steroid secretion are unknown, but may include a further compromise of adult stature.
-
PRECAUTIONS
Patients with known allergies to benzyl alcohol, an ingredient of the vehicle of LUPRON INJECTION, may present symptoms of hypersensitivity, usually local, in the form of erythema and induration at the injection site.
Information for Parents
Prior to starting therapy with LUPRON INJECTION, the parent or guardian must be aware of the importance of continuous therapy. Adherence to daily drug administration schedules must be accepted if therapy is to be successful. Irregular dosing could restart the maturation process.
- During the first 2 months of therapy, a female may experience menses or spotting. If bleeding continues beyond the second month, notify the physician.
- Any irritation at the injection site should be reported to the physician immediately. If the child has experienced an allergic reaction to other drugs like LUPRON, this drug should not be used.
- Report any unusual signs or symptoms to the physician, like continued pubertal changes, substantial mood swings or behavioral changes.
Laboratory Tests
Response to leuprolide acetate should be monitored 1-2 months after the start of therapy with a GnRH stimulation test and sex steroid levels. Measurement of bone age for advancement should be done every 6-12 months.
Sex steroids may increase or rise above prepubertal levels if the dose is inadequate (see WARNINGS section). Once a therapeutic dose has been established, gonadotropin and sex steroid levels will decline to prepubertal levels.
Drug/Laboratory Test Interactions
Administration of leuprolide acetate in therapeutic doses results in suppression of the pituitary-gonadal system. Normal function is usually restored within 4 to 12 weeks after treatment is discontinued.
Carcinogenesis, Mutagenesis, Impairment of Fertility
A two-year carcinogenicity study was conducted in rats and mice. In rats, a dose-related increase of benign pituitary hyperplasia and benign pituitary adenomas was noted at 24 months when the drug was administered subcutaneously at high daily doses of 0.6 to 4 mg/kg (>100 times the clinical doses of 7.5 to 15 mg/month based on body surface area). There was a significant but not dose-related increase of pancreatic islet-cell adenomas in females and of testes interstitial cell adenomas in males (highest incidence in the low dose group). In mice, no leuprolide acetate-induced tumors or pituitary abnormalities were observed at daily dose as high as 60 mg/kg (>5000 times the clinical doses based on body surface area). Adult patients have been treated with leuprolide acetate for up to three years with doses as high as 10 mg/day and for two years with doses as high as 20 mg/day without demonstrable pituitary abnormalities.
Although no clinical studies have been completed in children to assess the full reversibility of fertility suppression, animal studies (prepubertal and adult rats and monkeys) with leuprolide acetate and other GnRH analogs have shown functional recovery. However, following a study with leuprolide acetate, immature male rats demonstrated tubular degeneration in the testes even after a recovery period. In spite of the failure to recover histologically, the treated males proved to be as fertile as the controls. Also, no histologic changes were observed in the female rats following the same protocol. In both sexes, the offspring of the treated animals appeared normal. The effect of the treatment of the parents on the reproductive performance of the F1 generation was not tested. The clinical significance of these findings is unknown.
Pregnancy
Teratogenic Effects
Pregnancy Category X
(see CONTRAINDICATIONS section)
When administered on day 6 of pregnancy at test dosages of 0.00024, 0.0024, and 0.024 mg/kg (1/1200 to 1/12 the human pediatric dose) to rabbits, LUPRON produced a dose-related increase in major fetal abnormalities. Similar studies in rats failed to demonstrate an increase in fetal malformations. There was increased fetal mortality and decreased fetal weights with the two higher doses of LUPRON in rabbits and with the highest dose in rats.
-
ADVERSE REACTIONS
Clinical Trials
Potential exacerbation of signs and symptoms during the first few weeks of treatment (see PRECAUTIONS section) is a concern in patients with rapidly advancing central precocious puberty.
In two studies of children with central precocious puberty, in 2% or more of the patients receiving the drug, the following adverse reactions were reported to have a possible or probable relationship to drug as ascribed by the treating physician. Reactions considered not drug related are excluded.
Number of Patients
N = 395 (Percent)Body as a Whole General Pain 7 (2) Integumentary System Acne/Seborrhea 7 (2) Injection Site Reactions Including Abscess 21 (5) Rash Including Erythema Multiforme 8 (2) Urogenital System Vaginitis/Bleeding/ Discharge 7 (2) In those same studies, the following adverse reactions were reported in less than 2% of the patients.
Body as a Whole - Body Odor, Fever, Headache, Infection; Cardiovascular System - Syncope, Vasodilation; Digestive System - Dysphagia, Gingivitis, Nausea/Vomiting; Endocrine System - Accelerated Sexual Maturity; Metabolic and Nutritional Disorders - Peripheral Edema, Weight Gain; Nervous System - Nervousness, Personality Disorder, Somnolence, Emotional Lability; Respiratory System - Epistaxis; Integumentary System - Alopecia, Skin Striae; Urogenital System - Cervix Disorder, Gynecomastia/Breast Disorders, Urinary Incontinence.
Postmarketing
During postmarketing surveillance, which includes other dosage forms and other patient populations, the following adverse events were reported.
Symptoms consistent with an anaphylactoid or asthmatic process have been rarely (incidence rate of about 0.002%) reported. Rash, urticaria, and photosensitivity reactions have also been reported. Localized reactions including induration and abscess have been reported at the site of injection. Symptoms consistent with fibromyalgia (e.g., joint and muscle pain, headaches, sleep disorders, gastrointestinal distress, and shortness of breath) have been reported individually and collectively.
Cardiovascular System – Hypotension, Pulmonary embolism; Gastrointestinal System – Hepatic dysfunction; Hemic and Lymphatic System – Decreased WBC; Integumentary System – Hair growth; Central/Peripheral Nervous System – Peripheral neuropathy, Spinal fracture/paralysis, Hearing disorder; Miscellaneous – Hard nodule in throat, Weight gain, Increased uric acid; Musculoskeletal System – Tenosynovitis-like symptoms; Respiratory System – Respiratory disorders; Urogenital System – Prostate pain.
Changes in Bone Density: Decreased bone density has been reported in the medical literature in men who have had orchiectomy or who have been treated with an LH-RH agonist analog. In a clinical trial, 25 men with prostate cancer, 12 of whom had been treated previously with leuprolide acetate for at least six months, underwent bone density studies as a result of pain. The leuprolide-treated group had lower bone density scores than the nontreated control group. The effects on bone density in children are unknown.
Pituitary apoplexy: During post-marketing surveillance, rare cases of pituitary apoplexy (a clinical syndrome secondary to infarction of the pituitary gland) have been reported after the administration of gonadotropin-releasing hormone agonists. In a majority of these cases, a pituitary adenoma was diagnosed, with a majority of pituitary apoplexy cases occurring within 2 weeks of the first dose, and some within the first hour. In these cases, pituitary apoplexy has presented as sudden headache, vomiting, visual changes, ophthalmoplegia, altered mental status, and sometimes cardiovascular collapse. Immediate medical attention has been required.
See other LUPRON INJECTION and LUPRON DEPOT package inserts for adverse events reported in other patient populations.
-
OVERDOSAGE
In rats, subcutaneous administration of 125 to 250 times the recommended human pediatric dose, expressed on a per body weight basis, resulted in dyspnea, decreased activity, and local irritation at the injection site. There is no evidence at present that there is a clinical counterpart of this phenomenon. In early clinical trials using leuprolide acetate in adult patients, doses as high as 20 mg/day for up to two years caused no adverse effects differing from those observed with the 1 mg/day dose.
-
DOSAGE AND ADMINISTRATION
LUPRON INJECTION can be administered by a patient/parent or health care professional.
The dose of LUPRON INJECTION must be individualized for each child. The dose is based on a mg/kg ratio of drug to body weight. Younger children require higher doses on a mg/kg ratio.
After 1-2 months of initiating therapy or changing doses, the child must be monitored with a GnRH stimulation test, sex steroids, and Tanner staging to confirm downregulation. Measurements of bone age for advancement should be monitored every 6-12 months. The dose should be titrated upward until no progression of the condition is noted either clinically and/or by laboratory parameters.
The first dose found to result in adequate downregulation can probably be maintained for the duration of therapy in most children. However, there are insufficient data to guide dosage adjustment as patients move into higher weight categories after beginning therapy at very young ages and low dosages. It is recommended that adequate downregulation be verified in such patients whose weight has increased significantly while on therapy.
As with other drugs administered by injection, the injection site should be varied periodically.
Discontinuation of LUPRON INJECTION should be considered before age 11 for females and age 12 for males.
The recommended starting dose is 50 mcg/kg/day administered as a single subcutaneous injection. If total downregulation is not achieved, the dose should be titrated upward by 10 mcg/kg/day. This dose will be considered the maintenance dose.
Follow the pictorial directions on the reverse side of this package insert for administration.
NOTE: As with other parenteral products, inspect the solution for discoloration and particulate matter before each use.
-
HOW SUPPLIED
LUPRON INJECTION (leuprolide acetate) is a sterile solution supplied in a 2.8 mL multiple-dose vial. The vial is packaged as follows:
- 14 Day Patient Administration Kit with 14 disposable syringes and 28 alcohol swabs, NDC 0300-3612-28.
- Six-vial carton, NDC 0300-3612-24.
- Store below 77°F (25°C). Do not freeze. Protect from light; store vial in carton until use.
- Use the syringes supplied with LUPRON INJECTION. Insulin syringes may be substituted for use with LUPRON INJECTION.
U.S. Patent Nos. 4,005,063; 4,005,194.
- REFERENCES
- SPL UNCLASSIFIED SECTION
- ADMINISTERING THE INJECTION
-
SPL UNCLASSIFIED SECTION
TAP Pharmaceutical Products Inc.
Lake Forest, IL 60045, U.S.A.(No. 3612)
03-A066 R7; December 2007© 1993–2007 TAP Pharmaceutical Products Inc.
Provided as an educational service by
TAP Pharmaceuticals Inc.TAP Pharmaceutical Products Inc.
Lake Forest, IL 60045, U.S.A.[Bar code 128 of commodity number]
-
SUPPLEMENTAL PATIENT MATERIAL
ADMINISTERING THE INJECTION
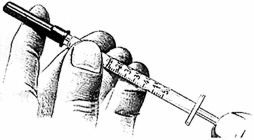
- 1.
-
Wash hands thoroughly.
- 2.
-
Check the liquid in the container. It should look clear. DO NOT USE if it is not clear or if it has particles in it. If using a new bottle, flip off the plastic cover to expose the grey rubber stopper. Use an alcohol swab to cleanse the metal ring and rubber stopper on medication bottle every day, just before you use it.
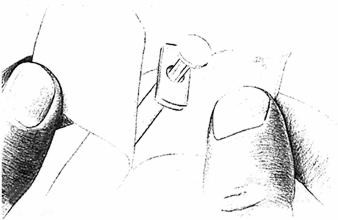
- 3.
-
Remove outer wrapping from one syringe.
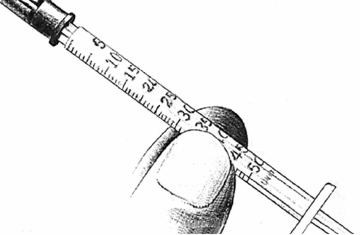
- 4.
-
Pull the syringe plunger back until its tip is at the proper mark.
- 5.
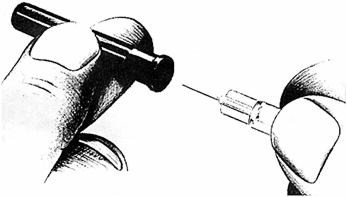
-
Uncover needle. Do not touch the needle.
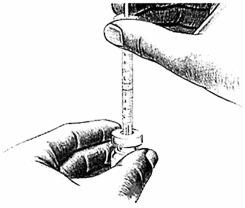
- 6.
-
Place the bottle on a clean, flat surface and push the needle through the center of the rubber stopper on the bottle. Push the plunger all the way in to inject air into the bottle.
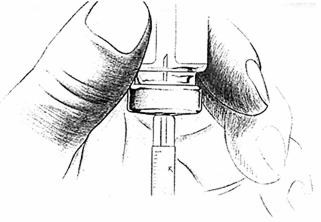
- 7.
-
Keep the needle in the bottle.
Lift the bottle and turn it straight upside down.
Check to see that the needle tip is in the liquid.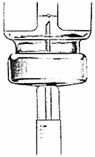
- 8.
-
With the needle tip in the liquid, slowly pull back the plunger until syringe fills to the proper mark.
If any bubbles appear in the syringe, remove them by pushing the plunger up slowly.
With the needle tip still in the liquid, pull the plunger until it is once more at the proper mark.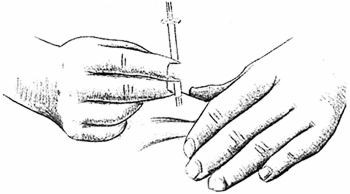
- 9.
-
Choose a different injection site each day.
Cleanse the injection site with a new alcohol swab.
Hold the skin the way you were instructed.
Slide the needle quickly all the way through the skin, into the subcutaneous tissue, at a 90° angle.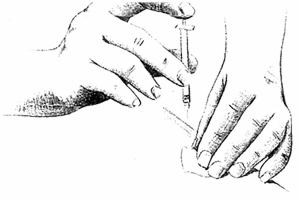
- 10.
-
Push the plunger to inject the medication.
Withdraw the needle at the same angle it was inserted (90°).
Wipe the skin with an alcohol swab.
- 11.
-
Dispose of the syringe and alcohol swabs as you were instructed.
Remember: use the disposable syringe only once.
-
INGREDIENTS AND APPEARANCE
LUPRON
leuprolide acetate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0300-3612 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0300-3612-28 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 2.8 mL Part 2 28 28 Part 1 of 2 LUPRON
leuprolide acetate injection, solutionProduct Information Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength leuprolide acetate (UNII: 37JNS02E7V) (leuprolide - UNII:EFY6W0M8TG) 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength sodium chloride (UNII: 451W47IQ8X) 6.3 mg in 1 mL benzyl alcohol (UNII: LKG8494WBH) 9 mg in 1 mL water (UNII: 059QF0KO0R) sodium hydroxide (UNII: 55X04QC32I) acetic acid, () Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0300-3612-28 1 in 1 CARTON 1 2.8 mL in 1 VIAL, MULTI-DOSE Part 2 of 2 ALCOHOL
alcohol swabProduct Information Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength Alcohol (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 PACKET LUPRON
leuprolide acetate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0300-3612 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength leuprolide acetate (UNII: 37JNS02E7V) (leuprolide - UNII:EFY6W0M8TG) 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength sodium chloride (UNII: 451W47IQ8X) 6.3 mg in 1 mL benzyl alcohol (UNII: LKG8494WBH) 9 mg in 1 mL water (UNII: 059QF0KO0R) sodium hydroxide (UNII: 55X04QC32I) acetic acid (UNII: Q40Q9N063P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0300-3612-24 6 in 1 CARTON 1 1 in 1 CARTON 1 2.8 mL in 1 VIAL, MULTI-DOSE Labeler - TAP Pharmaceutical Products Inc.