Label: PROVENGE- sipuleucel-t injection
- NDC Code(s): 30237-8900-5, 30237-8900-6
- Packager: Dendreon Pharmaceuticals LLC
- Category: CELLULAR THERAPY
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated October 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PROVENGE ® (sipuleucel-T) safely and effectively. See full prescribing information for PROVENGE.
PROVENGE ® (sipuleucel-T)
Suspension for Intravenous Infusion
Initial U.S. Approval: 2010RECENT MAJOR CHANGES
INDICATIONS AND USAGE
PROVENGE is an autologous cellular immunotherapy indicated for the treatment of asymptomatic or minimally symptomatic metastatic castrate-resistant (hormone-refractory) prostate cancer. ( 1)
DOSAGE AND ADMINISTRATION
For autologous use only.
For intravenous use only.
- Administer 3 doses at approximately 2-week intervals. ( 2.1)
- Premedicate patients with oral acetaminophen and an antihistamine such as diphenhydramine. ( 2.2)
- Before infusion, confirm that the patient's identity matches the patient identifiers on the infusion bag. ( 2.2)
- Infuse PROVENGE intravenously over a period of approximately 60 minutes. Do Not Use a Cell Filter. ( 2.2)
- Interrupt or slow infusion for acute infusion reactions, depending on the severity of the reaction. ( 2.2)
DOSAGE FORMS AND STRENGTHS
Each dose of PROVENGE contains a minimum of 50 million autologous CD54 + cells activated with PAP-GM-CSF, suspended in 250 mL of Lactated Ringer's Injection, USP. ( 3)
CONTRAINDICATIONS
None. ( 4)
WARNINGS AND PRECAUTIONS
- Acute infusion reactions may occur. If reactions occur, decrease the rate or stop the infusion and administer appropriate medical treatment. ( 5.1)
- Syncope and hypotension have also been observed. Closely monitor patients with cardiac or pulmonary conditions. ( 5.1)
- PROVENGE should be used with caution in patients with risk factors for thromboembolic events. ( 5.2)
- PROVENGE is not tested for transmissible infectious diseases and may transmit diseases to health care professionals handling the product. Universal precautions should be followed. ( 5.4)
- Concomitant use of chemotherapy and immunosuppressive medications with PROVENGE has not been studied. ( 5.5)
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥ 15%) are chills, fatigue, fever, back pain, nausea, joint ache, and headache. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Dendreon Pharmaceuticals LLC at 1-877-336-3736 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Acute Infusion Reactions
5.2 Thromboembolic Events
5.3 Vascular Disorders
5.4 Handling Precautions for Control of Infectious Disease
5.5 Concomitant Chemotherapy or Immunosuppressive Therapy
5.6 Product Safety Testing
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.5 Geriatric
8.6 Race
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For autologous use only.
For intravenous use only.
2.1 Dose
Each dose of PROVENGE contains a minimum of 50 million autologous CD54 + cells activated with PAP-GM-CSF [ see Description ( 11) ].
The recommended course of therapy for PROVENGE is 3 complete doses, given at approximately 2-week intervals. In controlled clinical trials, the median dosing interval between infusions was 2 weeks (range 1 to 15 weeks); the maximum dosing interval has not been established.
If, for any reason, the patient is unable to receive a scheduled infusion of PROVENGE, the patient will need to undergo an additional leukapheresis procedure prior to continuing a course of treatment. Advise patients of this possibility prior to initiating treatment.
2.2 Administration
- Do not use PROVENGE until confirmation of product release is received from Dendreon.
- Dendreon will send the Final Product Disposition Notification form containing the patient identifiers, expiration date and time, and the disposition status (approved for infusion or rejected), to the infusion site. Infusion must begin prior to the expiration date and time indicated on the Final Product Disposition Notification form and Product Label. Do not use expired PROVENGE. Keep the sealed, patient-specific PROVENGE infusion bag within the insulated polyurethane container inside the outer cardboard shipping box until the time of administration.
- To minimize potential acute infusion reactions, premedicate the patients orally with acetaminophen and an antihistamine, such as diphenhydramine, approximately 30 minutes prior to administration of PROVENGE.
Administration steps:
- Remove the infusion bag from the insulated polyurethane container and inspect the bag for signs of leakage or external damage. Contents of the bag will be clear to opaque, with a white to red color, including shades of off-white, cream, light yellow and orange. Remove the infusion bag from the insulated polyurethane container and inspect the bag for signs of leakage or external damage. Contents of the bag will be clear to opaque, with a white to red color, including shades of off-white, cream, light yellow and orange.
- Gently mix and resuspend the contents of the bag, inspecting for clumps and clots. Small clumps of cellular material should disperse with gentle manual mixing. Do not administer if the bag leaks during handling, is damaged, or if clumps remain in the bag.
- Match the patient's identity with the patient identifiers on the Final Product Disposition Notification form and the PROVENGE infusion bag.
- Infuse the entire volume of the PROVENGE infusion bag intravenously over approximately 60 minutes. Do not use a cell filter.
- Observe the patient for acute infusion reactions for at least 30 minutes following each infusion.
- If acute infusion reactions occur, such as chills, fatigue, fever, nausea, and joint ache, interrupt or slow the infusion and administer appropriate medical treatment as needed. In controlled clinical trials, symptoms of acute infusion reactions were treated with acetaminophen, intravenous H1 and/or H2 blockers, and low-dose intravenous meperidine.
- If the infusion is interrupted, keep the PROVENGE infusion bag at room temperature.
- Do not resume infusion if the PROVENGE infusion bag has been at room temperature for more than 3 hours.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Acute Infusion Reactions
Acute infusion reactions (reported within 1 day of infusion) may occur and include nausea, vomiting, fatigue, fever, rigor or chills, respiratory events (dyspnea, hypoxia, and bronchospasm), syncope, hypotension, hypertension, and tachycardia. Acute infusion reactions (reported within 1 day of infusion) may occur and include nausea, vomiting, fatigue, fever, rigor or chills, respiratory events (dyspnea, hypoxia, and bronchospasm), syncope, hypotension, hypertension, and tachycardia.
In controlled clinical trials, 71.2% of patients in the PROVENGE group developed an acute infusion reaction. The most common events (≥ 20%) were chills, fever, and fatigue. In 95.1% of patients reporting acute infusion reactions, the reactions were mild or moderate. Fevers and chills generally resolved within 2 days (71.9% and 89%, respectively). In controlled clinical trials, 71.2% of patients in the PROVENGE group developed an acute infusion reaction. The most common events (≥ 20%) were chills, fever, and fatigue. In 95.1% of patients reporting acute infusion reactions, the reactions were mild or moderate. Fevers and chills generally resolved within 2 days (71.9% and 89%, respectively).
In controlled clinical trials, severe (Grade 3) acute infusion reactions were reported in 3.5% of patients in the PROVENGE group. Reactions included chills, fever, fatigue, asthenia, dyspnea, hypoxia, bronchospasm, dizziness, headache, hypertension, muscle ache, nausea, and vomiting. The incidence of severe events was greater following the second infusion (2.1% vs. 0.8% following the first infusion), and decreased to 1.3% following the third infusion. Some (1.2%) patients in the PROVENGE group were hospitalized within 1 day of infusion for management of acute infusion reactions. No Grade 4 or 5 acute infusion reactions were reported in patients in the PROVENGE group. In controlled clinical trials, severe (Grade 3) acute infusion reactions were reported in 3.5% of patients in the PROVENGE group. Reactions included chills, fever, fatigue, asthenia, dyspnea, hypoxia, bronchospasm, dizziness, headache, hypertension, muscle ache, nausea, and vomiting. The incidence of severe events was greater following the second infusion (2.1% vs. 0.8% following the first infusion), and decreased to 1.3% following the third infusion. Some (1.2%) patients in the PROVENGE group were hospitalized within 1 day of infusion for management of acute infusion reactions. No Grade 4 or 5 acute infusion reactions were reported in patients in the PROVENGE group.
Closely monitor patients with cardiac or pulmonary conditions. In the event of an acute infusion reaction, decrease the infusion rate or stop the infusion, depending on the severity of the reaction. Administer appropriate medical treatment as needed. [ ] Closely monitor patients with cardiac or pulmonary conditions. In the event of an acute infusion reaction, decrease the infusion rate or stop the infusion, depending on the severity of the reaction. Administer appropriate medical treatment as needed. [ See Dosage and Administration ( 2.2) ]
5.2 Thromboembolic Events
Thromboembolic events, including deep venous thrombosis and pulmonary embolism, can occur following infusion of PROVENGE. The clinical significance and causal relationship are uncertain. Most patients had multiple risk factors for these events. PROVENGE should be used with caution in patients with risk factors for thromboembolic events. Thromboembolic events, including deep venous thrombosis and pulmonary embolism, can occur following infusion of PROVENGE. The clinical significance and causal relationship are uncertain. Most patients had multiple risk factors for these events. PROVENGE should be used with caution in patients with risk factors for thromboembolic events.
5.3 Vascular Disorders
: In controlled clinical trials, cerebrovascular events (hemorrhagic and ischemic strokes) were observed in 3.5% of patients in the PROVENGE group compared with 2.6% of patients in the control group. In the postmarketing setting, cerebrovascular events, including transient ischemic attacks, have been observed following infusion of Provenge. The clinical significance and causal relationship are uncertain. Most patients had multiple risk factors for these events. Cerebrovascular disease: In controlled clinical trials, cerebrovascular events (hemorrhagic and ischemic strokes) were observed in 3.5% of patients in the PROVENGE group compared with 2.6% of patients in the control group. In the postmarketing setting, cerebrovascular events, including transient ischemic attacks, have been observed following infusion of Provenge. The clinical significance and causal relationship are uncertain. Most patients had multiple risk factors for these events.
: In controlled clinical trials, myocardial infarctions were observed in 0.8% of patients in the PROVENGE group compared with 0.3% of patients in the control group. In the postmarketing setting, myocardial infarctions have been observed following infusion of Provenge. The clinical significance and causal relationship are uncertain. Most patients had multiple risk factors for these events. Cardiovascular disorders: In controlled clinical trials, myocardial infarctions were observed in 0.8% of patients in the PROVENGE group compared with 0.3% of patients in the control group. In the postmarketing setting, myocardial infarctions have been observed following infusion of Provenge. The clinical significance and causal relationship are uncertain. Most patients had multiple risk factors for these events.
5.4 Handling Precautions for Control of Infectious Disease
PROVENGE is not tested for transmissible infectious diseases. Therefore, patient leukapheresis material and PROVENGE may carry the risk of transmitting infectious diseases to health care professionals handling the product. Accordingly, health care professionals should employ universal precautions when handling leukapheresis material or PROVENGE.
5.5 Concomitant Chemotherapy or Immunosuppressive Therapy
Use of either chemotherapy or immunosuppressive agents (such as systemic corticosteroids) given concurrently with the leukapheresis procedure or PROVENGE has not been studied. PROVENGE is designed to stimulate the immune system, and concurrent use of immune-suppressive agents may alter the efficacy and/or safety of PROVENGE. Therefore, evaluate patients carefully to determine whether it is medically appropriate to reduce or discontinue immunosuppressive agents prior to treatment with PROVENGE.
5.6 Product Safety Testing
PROVENGE is released for infusion based on the microbial and sterility results from several tests: microbial contamination determination by Gram stain, endotoxin content, and in-process sterility with a 2-day incubation to determine absence of microbial growth. The final (7-day incubation) sterility test results are not available at the time of infusion. If the sterility results become positive for microbial contamination after PROVENGE has been approved for infusion, Dendreon will notify the treating physician. Dendreon will attempt to identify the microorganism, perform antibiotic sensitivity testing on recovered microorganisms, and communicate the results to the treating physician. Dendreon may request additional information from the physician in order to determine the source of contamination.
-
6 ADVERSE REACTIONS
The most common adverse reactions reported in clinical trials (≥ 15% of patients receiving PROVENGE) were chills, fatigue, fever, back pain, nausea, joint ache, and headache.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety evaluation of PROVENGE is based on 601 prostate cancer patients in the PROVENGE group who underwent at least 1 leukapheresis procedure in four randomized, controlled clinical trials. The control group (n = 303) received non-activated autologous peripheral blood mononuclear cells. Patients received 3 infusions of PROVENGE or control every other week over a period of 4 weeks.
Almost all (98.3%) patients in the PROVENGE group and 96% in the control group reported an adverse event. In 67.4% of patients in the PROVENGE group, these adverse events were mild or moderate in severity. Severe (Grade 3) and life-threatening (Grade 4) adverse events were reported in 23.6% and 4% of patients in the PROVENGE group compared with 25.1% and 3.3% of patients in the control group. Fatal (Grade 5) adverse events were reported in 3.3% of patients in the PROVENGE group compared with 3.6% of patients in the control group. The most common (≥ 2%) Grade 3-5 adverse events reported in the PROVENGE group were back pain and chills.
Serious adverse events were reported in 24% of patients in the PROVENGE group and 25.1% of patients in the control group. Serious adverse events in the PROVENGE group included acute infusion reactions [ see Warnings and Precautions ( 5.1) ], cerebrovascular events [ see Warnings and Precautions ( 5.3) ], and single case reports of eosinophilia, rhabdomyolysis, myasthenia gravis, myositis, and tumor flare.
PROVENGE was discontinued in 1.5% of patients in Study 1 due to adverse events. Some patients who required central venous catheters for treatment with PROVENGE developed infections, including sepsis. A small number of these patients discontinued treatment as a result. Monitoring for infectious sequelae in patients with central venous catheters is recommended.
Each dose of PROVENGE requires a standard leukapheresis procedure approximately 3 days prior to the infusion. Adverse events that were reported ≤ 1 day following a leukapheresis procedure in ≥ 5% of patients in controlled clinical trials included citrate toxicity (14.2%), oral paresthesia (12.6%), paresthesia (11.4%), and fatigue (8.3%).
Table 1 provides the frequency and severity of adverse events reported in ≥ 5% of patients in the PROVENGE group of randomized, controlled trials of men with prostate cancer. The population included 485 patients with metastatic castrate-resistant prostate cancer and 116 patients with non-metastatic androgen-dependent prostate cancer who were scheduled to receive 3 infusions of PROVENGE at approximately 2-week intervals. The population was age 40 to 91 years (median 70 years), and 90.6% of patients were Caucasian.
Table 1 Incidence of Adverse Events Occurring in ≥ 5% of Patients Randomized to PROVENGE PROVENGE
(N = 601)Control*
(N = 303)All Grades
n (%)Grade 3-5
n (%)All Grades
n (%)Grade 3-5
n (%)Any Adverse Event 591 (98.3) 186 (30.9) 291 (96.0) 97 (32.0) * Control group received non-activated autologous peripheral blood mononuclear cells.
Chills 319 (53.1) 13 (2.2) 33 (10.9) 0 (0.0) Fatigue 247 (41.1) 6 (1.0) 105 (34.7) 4 (1.3) Fever 188 (31.3) 6 (1.0) 29 (9.6) 3 (1.0) Back pain 178 (29.6) 18 (3.0) 87 (28.7) 9 (3.0) Nausea 129 (21.5) 3 (0.5) 45 (14.9) 0 (0.0) Joint ache 118 (19.6) 11 (1.8) 62 (20.5) 5 (1.7) Headache 109 (18.1) 4 (0.7) 20 (6.6) 0 (0.0) Citrate toxicity 89 (14.8) 0 (0.0) 43 (14.2) 0 (0.0) Paresthesia 85 (14.1) 1 (0.2) 43 (14.2) 0 (0.0) Vomiting 80 (13.3) 2 (0.3) 23 (7.6) 0 (0.0) Anemia 75 (12.5) 11 (1.8) 34 (11.2) 7 (2.3) Constipation 74 (12.3) 1 (0.2) 40 (13.2) 3 (1.0) Pain 74 (12.3) 7 (1.2) 20 (6.6) 3 (1.0) Paresthesia oral 74 (12.3) 0 (0.0) 43 (14.2) 0 (0.0) Pain in extremity 73 (12.1) 5 (0.8) 40 (13.2) 1 (0.3) Dizziness 71 (11.8) 2 (0.3) 34 (11.2) 0 (0.0) Muscle ache 71 (11.8) 3 (0.5) 17 (5.6) 0 (0.0) Asthenia 65 (10.8) 6 (1.0) 20 (6.6) 2 (0.7) Diarrhea 60 (10.0) 1 (0.2) 34 (11.2) 3 (1.0) Influenza-like illness 58 (9.7) 0 (0.0) 11 (3.6) 0 (0.0) Musculoskeletal pain 54 (9.0) 3 (0.5) 31 (10.2) 3 (1.0) Dyspnea 52 (8.7) 11 (1.8) 14 (4.6) 3 (1.0) Edema peripheral 50 (8.3) 1 (0.2) 31 (10.2) 1 (0.3) Hot flush 49 (8.2) 2 (0.3) 29 (9.6) 1 (0.3) Hematuria 46 (7.7) 6 (1.0) 18 (5.9) 3 (1.0) Muscle spasms 46 (7.7) 2 (0.3) 17 (5.6) 0 (0.0) Hypertension 45 (7.5) 3 (0.5) 14 (4.6) 0 (0.0) Anorexia 39 (6.5) 1 (0.2) 33 (10.9) 3 (1.0) Bone pain 38 (6.3) 4 (0.7) 22 (7.3) 3 (1.0) Upper respiratory tract infection 38 (6.3) 0 (0.0) 18 (5.9) 0 (0.0) Insomnia 37 (6.2) 0 (0.0) 22 (7.3) 1 (0.3) Musculoskeletal chest pain 36 (6.0) 2 (0.3) 23 (7.6) 2 (0.7) Cough 35 (5.8) 0 (0.0) 17 (5.6) 0 (0.0) Neck pain 34 (5.7) 3 (0.5) 14 (4.6) 2 (0.7) Weight decreased 34 (5.7) 2 (0.3) 24 (7.9) 1 (0.3) Urinary tract infection 33 (5.5) 1 (0.2) 18 (5.9) 2 (0.7) Rash 31 (5.2) 0 (0.0) 10 (3.3) 0 (0.0) Sweating 30 (5.0) 1 (0.2) 3 (1.0) 0 (0.0) Tremor 30 (5.0) 0 (0.0) 9 (3.0) 0 (0.0) 6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of PROVENGE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Nervous system disorders: syncope, transient ischemic attack, strokes [see Warnings and Precautions ( 5.1, 5.3)]
- Vascular disorders: hypotension [see Warnings and Precautions ( 5.1)]
- Cardiovascular disorders: myocardial infarction [see Warnings and Precautions ( 5.3)]
- Thromboembolic disorders: deep venous thrombosis and pulmonary embolism [see Warnings and Precautions ( 5.2)]
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.5 Geriatric
In controlled clinical trials, 72.9% of patients (438 of 601) in the PROVENGE group were ≥ 65 years of age. There were no apparent differences in the safety of PROVENGE between patients ≥ 65 years of age and younger patients.
In a survival analysis of the controlled clinical trials of PROVENGE in metastatic castrate-resistant prostate cancer, 78.3% of randomized patients (382 of 488) were ≥ 65 years of age. The median survival of patients in the PROVENGE group ≥ 65 years of age was 23.4 months (95% confidence interval 22.0, 27.1), compared with 17.3 months in the control group (95% confidence interval: 13.5, 21.5).
-
10 OVERDOSAGE
Each PROVENGE infusion comprises the maximum number of cells that can be manufactured from a single leukapheresis procedure. The number of cells in PROVENGE does not exceed the number of cells collected from the leukapheresis. There are no known instances of overdosage from either a single infusion or a full course of therapy with PROVENGE.
-
11 DESCRIPTION
PROVENGE (sipuleucel-T) is an autologous cellular immunotherapy available as a suspension for intravenous infusion. PROVENGE consists of autologous peripheral blood mononuclear cells, including antigen presenting cells (APCs), that have been activated during a defined culture period with a recombinant human protein, PAP-GM-CSF, consisting of prostatic acid phosphatase (PAP), an antigen expressed in prostate cancer tissue, linked to granulocyte-macrophage colony-stimulating factor (GM-CSF), an immune cell activator. Each dose of PROVENGE contains a minimum of 50 million autologous CD54 + cells activated with PAP-GM-CSF, suspended in 250 mL of Lactated Ringer's Injection, USP.
The active components of PROVENGE are autologous APCs and PAP-GM-CSF. During culture, the recombinant antigen can bind to and be processed by APCs into smaller protein fragments. The recombinant antigen is designed to target APCs, and may help direct the immune response to PAP. Minimal residual levels of the intact PAP-GM-CSF are detectable in the final PROVENGE product.
The patient's peripheral blood mononuclear cells are obtained via a standard leukapheresis procedure approximately 3 days prior to the infusion date. Due to the autologous nature of PROVENGE, it is important that the patient and physician adhere to the personalized leukapheresis and infusion schedules.
The cellular composition of PROVENGE is dependent on the composition of cells obtained from the patient's leukapheresis. In addition to APCs, the final product contains T cells, B cells, natural killer (NK) cells, and other cells. The number of cells present and the cellular composition of each PROVENGE dose will vary.
The potency of PROVENGE is in part determined by measuring the increased expression of the CD54 molecule, also known as ICAM-1, on the surface of APCs after culture with PAP-GM-CSF. CD54 is a cell surface molecule that plays a role in the immunologic interactions between APCs and T cells, and is considered a marker of immune cell activation.
In-process and final sterility tests are initiated prior to shipping, but the final results are not available for up to 7 days. PROVENGE is released for shipping based on acceptable results from 2-day incubation of the in-process sterility test.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
PROVENGE is classified as an autologous cellular immunotherapy. While the precise mechanism of action is unknown, PROVENGE is designed to induce an immune response targeted against PAP, an antigen expressed in most prostate cancers. During ex vivo culture with PAP-GM-CSF, APCs take up and process the recombinant target antigen into small peptides that are then displayed on the APC surface.
In Study 1, 237 out of the 512 patients randomized were evaluated for the development of humoral and T cell immune responses (proliferative and gamma-interferon (γIFN) ELISPOT) to the target antigens at Baseline, and at Weeks 6, 14, and 26. Antibody (IgM and IgG) responses against PAP-GM-CSF and PAP antigen alone were observed through the follow-up period in the PROVENGE group. Neutralizing antibody responses to GM-CSF were transient. T cell proliferative and γIFN ELISPOT responses to PAP-GM-CSF fusion protein were observed in cells collected from peripheral blood of patients through the follow-up period in the PROVENGE treatment group but not in controls. In some patients a response to PAP antigen alone was observed. No conclusions could be made regarding the clinical significance of the observed immune responses.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The effect of PROVENGE on patients with metastatic castrate-resistant (hormone-refractory) prostate cancer was studied in three similar randomized, double-blind, placebo-controlled, multicenter trials. Following randomization, patients from both treatment groups underwent a series of 3 leukapheresis procedures (at approximately Weeks 0, 2, and 4). Each leukapheresis was followed approximately 3 days later by infusion of PROVENGE or control. The control was autologous peripheral blood mononuclear cells that had not been activated [ see Description ( 11)]. Following disease progression, patients were treated at the physician's discretion with other anti-cancer interventions.
Study 1
Study 1 was a randomized, double-blind, placebo-controlled, multicenter trial in patients with asymptomatic or minimally symptomatic metastatic castrate-resistant (hormone-refractory) prostate cancer. Eligible patients had metastatic disease in the soft tissue and/or bone with current or historical evidence of disease progression concomitant with surgical or medical castration, as evidenced by progression of serum Prostate Specific Antigen (PSA) and/or bone or soft tissue disease. Exclusion criteria included visceral (liver, lung, or brain) metastases, moderate to severe prostate cancer-related pain, and use of narcotics for cancer-related pain.
A total of 512 patients were randomized in a 2:1 ratio to receive PROVENGE (n=341) or control (n=171). The median age was 71, and 90% of the patients were Caucasian. Thirty-five percent of patients had undergone radical prostatectomy, 54% had received local radiotherapy, and 82% had received combined androgen blockade. All patients had baseline testosterone levels < 50 ng/mL. Forty-eight percent of patients were receiving bisphosphonates, and 18% had received prior chemotherapy, including docetaxel. Eighty-two percent of patients had an ECOG performance status of 0; 58% had primary Gleason scores of four or more; 44% had bone and soft tissue disease; 48% had bone-only disease; 7% had soft tissue-only disease; and 43% had greater than ten bony metastases.
Supportive Studies
Study 2 was a randomized, double-blind, placebo-controlled, multicenter trial in patients with metastatic castrate-resistant prostate cancer and no cancer-related pain. The primary endpoint was time to disease progression; analysis of the primary endpoint did not reach statistical significance. All patients were to be followed for survival; however, the survival analysis was not pre-specified.
Summary of Study Results
Figure 1 and Table 2 present overall survival results observed in two randomized, Phase 3 studies of PROVENGE in men with metastatic castrate-resistant prostate cancer. The survival findings were consistent across multiple subgroups. Analyses of time to disease progression did not meet statistical significance in any Phase 3 study of PROVENGE.
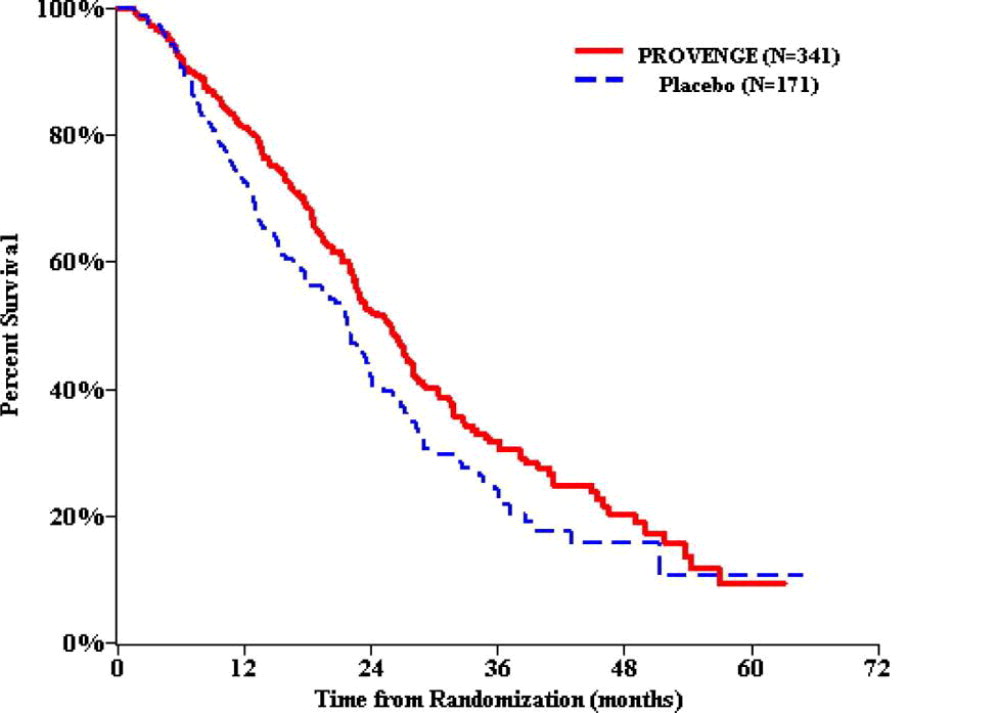
Figure 1 Kaplan-Meier Overall Survival Curve for Study 1
Table 2 Summary of Overall Survival (All Patients as Randomized) a Hazard ratio and p-value based on the Cox Model adjusted for PSA (ln) and LDH (ln) and stratified by bisphosphonate use, number of bone metastases, and primary Gleason grade.
b Hazard ratio based on the unadjusted Cox Model (not pre-specified).
c p-value based on a log-rank test (not pre-specified).
Abbreviations: CI = confidence interval.
Study 1 Study 2 PROVENGE
(N=341)Control
(N=171)PROVENGE (N=82) Control
(N=45)Overall Survival Median, months
(95% CI)25.8
(22.8, 27.7)21.7
(17.7, 23.8)25.9
(20.0, 32.4)21.4
(12.3, 25.8)Hazard Ratio
(95% CI)0.775 a (0.614, 0.979) 0.586 b (0.388, 0.884) p-value 0.032 a 0.010 c -
16 HOW SUPPLIED/STORAGE AND HANDLING
PROVENGE is a 250 mL suspension containing a minimum of 50 million autologous CD54 + cells activated with PAP-GM-CSF in Lactated Ringer's Injection, USP. It is supplied in a sealed infusion bag, labeled for the specific recipient.
NDC 30237-8900-6: One bag individually packed in a carton.
PROVENGE is shipped directly to the infusing provider in a cardboard shipping box with a special insulated polyurethane container inside. The insulated container and gel packs within the container are designed to maintain the appropriate transportation and storage temperature of PROVENGE until infusion.
- Upon receipt, open the outer cardboard shipping box to verify the product and patient-specific labels located on the top of the insulated container.
- Do not remove the insulated container from the shipping box, or open the lid of the insulated container, until the patient is ready for infusion.
-
17 PATIENT COUNSELING INFORMATION
- Advise the patient to read the FDA-approved patient labeling ( Patient Information).
- Inform the patient or caregiver about the following:
- The recommended course of therapy for PROVENGE is 3 complete doses. Each infusion of PROVENGE is preceded by a leukapheresis procedure approximately 3 days prior. It is important to maintain all scheduled appointments and arrive at each appointment on time because the leukapheresis and infusions must be appropriately spaced and the PROVENGE expiration time must not be exceeded.
- If the patient is unable to receive an infusion of PROVENGE, the patient will need to undergo an additional leukapheresis procedure if the treatment is to be continued.
- Counsel the patient on the importance of adhering to preparation instructions for the leukapheresis procedure, the possible side effects of leukapheresis, and post-procedure care.
- If the patient does not have adequate peripheral venous access to accommodate the leukapheresis procedure and infusion of PROVENGE, inform the patient about the need for a central venous catheter. Counsel the patient on the importance of catheter care. Instruct the patient to tell their doctor if they are experiencing fevers or any swelling or redness around the catheter site, because these symptoms could be signs of an infected catheter.
- Report signs and symptoms of acute infusion reactions such as fever, chills, fatigue, breathing problems, dizziness, high blood pressure, low blood pressure, lightheadedness, nausea, vomiting, headache, or muscle aches.
- Report any symptoms suggestive of a cardiac arrhythmia.
- Report any symptoms suggestive of cardiac ischemia.
- Report any symptoms suggestive of cerebral ischemia.
- Report any symptoms suggestive of deep vein thrombosis.
- Report any symptoms suggestive of pulmonary embolism.
- Inform their doctor if they are taking immunosuppressive agents.
For more information, please call the toll-free number: 1-877-336-3736.
Dendreon Pharmaceuticals LLC
Seal Beach, CA 90740
LBS-76022.04Rev. 07/2017
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
PROVENGE® (sipuleucel-T)
This leaflet is designed to help you understand treatment with PROVENGE (pronounced PROH-venj). The more you understand your treatment, the better you will be able to participate in your care. This leaflet does not take the place of talking with your doctor or healthcare professional about your medical condition or your treatment. If you have any questions, speak with your doctor.
What is PROVENGE?
PROVENGE is a prescription medicine that is used to treat certain patients with advanced prostate cancer. PROVENGE is made from your own immune cells.
What should I tell my doctor before getting PROVENGE?
Tell your doctor about all your medical problems, including:
- heart problems
- lung problems
- history of stroke
Tell your doctor about all the medicines you take, including prescription and nonprescription drugs, vitamins, and dietary supplements.
How will I get PROVENGE?
Since PROVENGE is made from your own immune cells, your cells will be collected approximately 3 days before each scheduled infusion of PROVENGE. You will need to go to a cell collection center for this collection. The collection is called “leukapheresis” (pronounced loo-kuh-fuh-REE-sis). Your collected cells are sent to a manufacturing center where they are mixed with a protein to make them ready for your infusion.
You will get PROVENGE in 3 intravenous infusions (put into your veins), about 2 weeks apart. Each infusion takes about 60 minutes. Following each infusion, you will be monitored for at least 30 minutes.
Your doctor will give you a schedule for your cell collection and infusion appointments. It is very important that you arrive on time for your appointments. If you miss an appointment and cannot be infused, your PROVENGE dose will not be usable. Your doctor will work with you to schedule a new appointment at the cell collection center. You may also get a new infusion appointment.
What are the possible or reasonably likely side effects of PROVENGE?
The most common side effects of PROVENGE include:
- chills
- fatigue
- fever
- back pain
- nausea
- joint ache
- headache
PROVENGE infusion can cause serious reactions.
Tell your doctor right away if
- you have breathing problems, chest pains, racing heart or irregular heartbeats, high or low blood pressure, dizziness, fainting, nausea, or vomiting after getting PROVENGE. Any of these may be signs of heart or lung problems.
- you develop numbness or weakness on one side of the body, decreased vision in one eye or difficulty speaking. Any of these may be signs of a stroke.
- you develop symptoms of thrombosis which may include: pain and/or swelling of an arm or leg with warmth over the affected area, discoloration of an arm or leg, unexplained shortness of breath, chest pain that worsens on deep breathing.
- you get a fever over 100ºF, or redness or pain at the infusion or collection sites. Any of these may be signs of infection.
Tell your doctor about any side effect that concerns you or does not go away.
These are not all the possible side effects of PROVENGE treatment. For more information, talk with your doctor.
What are the ingredients in PROVENGE?
The active components of PROVENGE are your own immune cells mixed with the other active component, a protein designed to produce an immune response to prostate cancer. The product is suspended in an infusion solution called Lactated Ringer's Injection, USP, an inactive ingredient.
If you would like more information about PROVENGE, talk with your doctor. You can also call toll-free 1-877-336-3736 or visit www.PROVENGE.com.
Dendreon Pharmaceuticals LLC
Seal Beach, CA 90740
LBS-76022.04
Rev. 07/2017 -
PRINCIPAL DISPLAY PANEL
Principal Display Panel – Carton Label
NDC 30237-8900-6
sipuleucel-T
PROVENGE ®
RX ONLY FOR AUTOLOGOUS USE ONLY
No U.S. standard of potency
CONTENTS: A minimum of 50 million autologous CD54 + cells activated with PAP-GM-CSF and suspended in Lactated Ringer's Injection, USP.
Manufactured by:
Dendreon Pharmaceuticals LLC Seal Beach, CA 90740
Phone: 877-256-4545
U.S. Lic. # 1749
No preservatives. Gently mix and re-suspend the contents of the bag. 76082.04
One autologous dose for infusion. See package insert for full prescribing information and instructions for administration.
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – Bag Label
NDC 30237-8900-5
sipuleucel-T
PROVENGE ®
RX ONLY FOR AUTOLOGOUS USE ONLY
No U.S. standard of potency
CONTENTS: A minimum of 50 million autologous CD54 + cells activated with PAP-GM-CSF and suspended in Lactated Ringer's Injection, USP.
Manufactured by:
Dendreon Pharmaceuticals LLC Seal Beach, CA 90740
Phone: 877-256-4545
U.S. Lic. # 1749
No preservatives. Gently mix and re-suspend the contents of the bag. 000074.01
One autologous dose for infusion. See package insert for full prescribing information and instructions for administration.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PROVENGE
sipuleucel-t injectionProduct Information Product Type CELLULAR THERAPY Item Code (Source) NDC:30237-8900 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SIPULEUCEL-T (UNII: 8Q622VDR18) (SIPULEUCEL-T - UNII:8Q622VDR18) SIPULEUCEL-T 50000000 Inactive Ingredients Ingredient Name Strength POTASSIUM CHLORIDE (UNII: 660YQ98I10) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:30237-8900-6 1 in 1 CARTON 1 NDC:30237-8900-5 1 in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125197 04/29/2010 Labeler - Dendreon Pharmaceuticals LLC (079879298) Registrant - Ann Davis (070452627) Establishment Name Address ID/FEI Business Operations Dendreon Pharmaceuticals LLC 079879298 manufacture(30237-8900) , analysis(30237-8900) , pack(30237-8900) , label(30237-8900) Establishment Name Address ID/FEI Business Operations Dendreon Pharmaceuticals LLC 968590237 manufacture(30237-8900) , analysis(30237-8900) , pack(30237-8900) , label(30237-8900)