Label: RADIOGARDASE- prussian blue insoluble capsules capsule
- NDC Code(s): 58060-002-02
- Packager: Heyl Chem.-pharm. Fabrik GmbH & Co. KG
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 7, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use RADIOGARDASE safely and effectively. See full prescribing information for RADIOGARDASE.
RADIOGARDASE (prussian blue insoluble) capsules, for oral use
Initial U.S. Approval: 2003INDICATIONS AND USAGE
Radiogardase is indicated for treatment of patients with known or suspected internal contamination with radioactive cesium and/or radioactive or non-radioactive thallium to increase their rates of elimination. ( 1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Capsules: 0.5 grams ( 3)
CONTRAINDICATIONS
None ( 4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reaction (incidence >24%) was constipation ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact info@heyl-berlin.de, Fax +49 30 817 4049 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2014
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Decontamination Procedures for Radioactive Cesium or Thallium Contamination
2.3 Recommended Dosage
2.4 Treatment of Radioactive Cesium Contamination
2.5 Treatment of Radioactive and Non-radioactive Thallium Contamination
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Increased Radiation Absorbed Dose to Gastrointestinal Mucosa
5.2 Constipation
5.3 Electrolyte Abnormalities
5.4 Blue Discoloration of Feces, Oral Mucosa, and Dentition
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Cesium-137 Contamination
14.2 Thallium Contamination
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
- Obtain quantitative baseline of the internalized contamination by radioactive cesium (137Cs) and/or thallium by appropriate whole-body counting and/or by bioassay (e.g., biodosimetry), or feces/urine samples, whenever possible prior to Radiogardase treatment.
- Initiate treatment with Radiogardase as soon as possible after contamination is suspected. Even when delayed, treatment with Radiogardase is effective and should not be withheld.
- Take Radiogardase capsules with food to stimulate excretion of cesium or thallium.
- In patients who cannot tolerate swallowing large numbers of capsules, open the capsules and mix with bland food or liquids.
2.2 Decontamination Procedures for Radioactive Cesium or Thallium Contamination
Prior to initiating treatment with Radiogardase, follow radioactive decontamination safety procedures including:
- Use appropriate radiation protective attire and closely monitor personnel and treatment area for radiation levels using radiation detection, indication, and computation devices (RADIAC) or thermal luminescent devices (TLD).
- Control spread of radiation contamination through the establishment of a patient decontamination area and a contaminated material disposal site (with proper labeling, handling, and disposal of contaminated material).
2.3 Recommended Dosage
- Adults and Adolescents: 3 grams (6 capsules) taken orally three times a day (a total daily dose of 9 grams)
- Pediatric Patients (2 – 12 years): 1 gram (2 capsules) taken orally three times a day (a total daily dose of 3 grams)
2.4 Treatment of Radioactive Cesium Contamination
- Anticipate that treatment with Radiogardase may last 30 days or longer.
- Base duration of Radiogardase treatment on weekly measurements of radioactivity in urine and fecal samples to monitor cesium elimination rate.
- Obtain weekly laboratory evaluations (complete blood count, serum chemistry and electrolytes).
2.5 Treatment of Radioactive and Non-radioactive Thallium Contamination
- Anticipate that treatment with Radiogardase may last 30 days or longer.
- For radioactive thallium:
- Base duration of Radiogardase treatment on weekly measurements of radioactivity inurine and fecal samples to monitor thallium elimination rate.
- Continue Radiogardase treatment until a 24-hour urine thallium test is normal (less than 5 micrograms per liter) and radiation level is acceptable.
- For non-radioactive thallium: Continue Radiogardase treatment until a 24-hour urine thallium test is normal (less than 5 micrograms per liter).
- Obtain weekly laboratory evaluations (complete blood count, serum chemistry and electrolytes).
- In cases of severe thallium intoxication, additional types of treatment may be necessary, such as:
- Induced emesis, followed by gastric intubation and lavage
- Forced diuresis until urinary thallium excretion is less than 1 mg/24 hours
- Charcoal hemoperfusion may be useful during the first 48 hours after thallium ingestion (biodistribution phase).
- Hemodialysis has also been reported to be effective in thallium intoxication.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Increased Radiation Absorbed Dose to Gastrointestinal Mucosa
Radiogardase can decrease gastrointestinal motility, thus slowing the transit time of radioactivity in the gastrointestinal tract. The slowed transit time can increase the radiation absorbed dose to the gastrointestinal mucosa.
5.2 Constipation
Radiogardase can cause constipation. Monitor and treat for signs and symptoms of constipation. Patients with disorders associated with decreased gastrointestinal motility are at higher risk.
5.3 Electrolyte Abnormalities
Radiogardase may bind to electrolytes found in the gastrointestinal tract. Hypokalemia, with serum potassium values of 2.5 – 2.9 (normal 3.5 – 5.0), was reported in 3 (7%) of 42 patients during treatement with Radiogardase. Monitor serum electrolytes during Radiogardase treatment, particularly when treating patients with pre-existing cardiac arrhythmias or electrolyte imbalances.
-
6 ADVERSE REACTIONS
Constipation was reported in 10 (24%) of 42 patients treated with Radiogardase. Severity of constipation was mild in 7 patients and moderate in 3 patients [see Warnings and Precautions (5.2)].
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
It is not known whether Radiogardase can cause fetal harm when administered to a pregnant woman or if it can affect reproduction capacity. Animal reproduction studies have not been conducted with prussian blue insoluble. However, since Radiogardase is not absorbed from the gastrointestinal tract, effects on the fetus are not expected.Radioactive cesium ( 137Cs) crosses the human placenta. One patient, contaminated with 0.005 mCi 137Cs during her 4 th month of pregnancy, was not treated with Radiogardase. At birth, the concentration of 137Cs was the same in the mother and the infant.
Thallium crosses the human placenta. Reported fetal effects include failure to thrive and death. The toxicity from untreated radioactive cesium or thallium exposure is greater than the potential reproductive toxicity of Radiogardase.
8.3 Nursing Mothers
Studies to determine if Radiogardase is excreted in human milk have not been conducted. Since Radiogardase is not absorbed from the gastrointestinal tract, its excretion in milk is unlikely. However, cesium and thallium are transmitted from mother to infant in breast milk. Women internally contaminated with cesium or thallium should not breastfeed.
8.4 Pediatric Use
Radioactive Cesium Contamination
The safety and efficacy of Radiogardase in the treatment of 137Cs in pediatric patients ages, 2 to 18 years old, was established from data from Radiogardase-treated pediatric patients exposed to 137Cs in the Goiânia, Brazil, contamination incident and from Radiogardase-treated adults exposed to 137Cs [see Clinical Studies (14.1)].Overall, 27 pediatric patients received Radiogardase in the range of 3 to 10 grams per day in divided doses (the maximum recommended adolescent dosage is 9 grams per day). Radiogardase treatment reduced the whole body effective half-life of 137Cs by 46% in adolescents and by 43% in children aged 4 to 12 years of age. In 12 patients for whom the rate of radiation elimination data are available, the rate was similar to that in adults treated with 3 grams three times daily and in pediatric patients treated with 1 gram three times daily. By body weight, the dose ranged from
0.32 gram/kg in the 12-year old patient (10 gram Radiogardase daily dose, 31 kg weight) to
0.21 gram/kg in the 4 year old patient (3 gram Radiogardase daily dose, 14 kg weight) [see Clinical Studies (14.1)] .
Pediatric patients aged 2 up to 4 years are expected to have biliary and gastrointestinal function that is comparable to that of a 4-year old.
The safety and efficacy of Radiogardase has not been established in the treatment of 137Cs contamination in pediatric patients 0 to 2 years old. There are differences in the developmental maturity of the biliary system and gastrointestinal tract of neonates and infants (0 – 2 years). The dosage-related adverse reactions of Radiogardase on an immature gastrointestinal tract are not known.
Radioactive and Non-Radioactive Thallium Contamination
The safety and efficacy of Radiogardase for the treatment of radioactive and non-radioactive thallium contamination in pediatric patients has not been established.8.5 Geriatric Use
The safety and efficacy of Radiogardase in patients aged 65 and over have not been evaluated, to determine whether they respond differently from younger subjects.. In general, elderly patients should be monitored closely, reflecting the greater frequency of decreased cardiac function and of concomitant disease or other drug therapy.
-
10 OVERDOSAGE
Based on reported adverse reactions and mechanism of action, possible overdosage symptoms may include constipation, obstruction, or severe decrease in electrolytes. Gastric distress was reported in 3 patients treated with 20 gram/day of Radiogardase (approximately 2.2 times the maximum recommended dosage). In these patients, the dose was reduced to 10 gram/day for continued treatment.
-
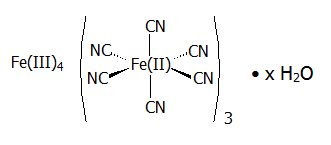
11 DESCRIPTION
Radiogardase (prussian blue insoluble) is a decorporation agent for oral use. Radiogardase capsules contain insoluble ferric hexacyanoferrate(II), with an empirical formula of Fe 4[Fe(CN) 6] 3 and a molecular weight of 859.3 Daltons. It is supplied as 0.5 gram of blue powder in gelatin capsules with 0 – 38 mg of microcrystalline cellulose. The dark blue capsule is imprinted with the light blue inscription:
 PB. The powder may vary from uniformly fine, dark granules to coarse light and dark-colored granules. The structural formula for prussian blue insoluble is shown below.
PB. The powder may vary from uniformly fine, dark granules to coarse light and dark-colored granules. The structural formula for prussian blue insoluble is shown below.
The crystal structure of prussian blue insoluble is a cubic lattice with the Fe II and Fe III atoms occupying the corners of the cube and the cyanide groups positioned on the sides.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Prussian blue insoluble, ferric hexacyanoferrate(II), acts by ion-exchange, adsorption, and mechanical trapping within the crystal structure, and has a high affinity for radioactive and non-radioactive cesium and thallium.
Prussian blue insoluble binds cesium and thallium isotopes in the gastrointestinal tract after these isotopes are ingested or excreted in the bile by the liver, thereby reducing gastrointestinal reabsorption (enterohepatic circulation). The rate of cesium and thallium elimination is proportional to the duration and dose of prussian blue insoluble.
12.2 Pharmacodynamics
Cesium-137 ( 137Cs)
137Cs has a physical half-life of 30 years, with a beta energy peak at 174.0 keV. Following entry into the blood, it is distributed uniformly through all body tissues. Approximately 10% of 137Cs is eliminated rapidly with a biological half-life of 2 days; 90% is eliminated more slowly, with a biological half-life of 110 days; and less than 1% of the 137Cs is retained with a biological half-life of about 500 days. 137Cs follows the movement of potassium and is excreted into the intestine, reabsorbed from the gastrointestinal (GI) tract into the blood, then to the bile, where it is excreted again into the GI tract by bile via enterohepatic circulation. Without Radiogardase treatment, about 80% of 137Cs is excreted through the kidneys and about 20% in the feces.Thallium-201 ( 201Tl)
Radioactive thallium ( 201Tl) has a physical half-life of 3 days with electron and photon emissions with a gamma energy peak at 167.4 keV. Non-radioactive thallium has a biological half-life of8 – 10 days.The physiologic transport of thallium follows the same route as potasium and is excreted by bile in enterohepatic circulation. Without Radiogardase treatment, the fecal to urine excretion ratio of thallium is approximately 2:1.
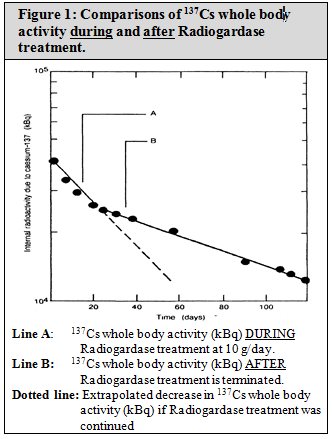
The results of fecal analysis from patients contaminated with 137Cs and treated with Radiogardase showed higher activities of 137Cs in feces, and the associated whole body radioactivity counts showed a more rapid rate of elimination from the body. The effectiveness of Radiogardase for one patient is shown in Figure 1. The whole body content of radioactive material of 137Cs in kilo-Bequerels (kBq) is shown on the y-axis. Time in days is on the x-axis. Line “A” represents the whole body activity of 137Cs during prussian blue insoluble treatment at 10 g/day. The dotted line represents extrapolation of the whole body activity if treatment was continued. Line “B” represents the whole body activity of 137Cs, after prussian blue insoluble was stopped.
12.3 Pharmacokinetics
Absorption/Elimination:
Prussian blue insoluble is not absorbed through the intact gastrointestinal wall. Its clearance from the body depends on the gastrointestinal tract transit time.Food Effects:
Food effect studies have not been conducted. In animal studies, Prussian blue insoluble was not significantly absorbed. Food may increase the effectiveness of prussian blue insoluble by stimulating bile secretion. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies have not been performed to evaluate the carcinogenic or mutagenic potential of prussian blue insoluble. No study on impairment of male or female fertility and reproductive performance has been conducted in animals.
13.2 Animal Toxicology and/or Pharmacology
Absorption/Elimination
In an animal study (pigs, n =38), after a single dose of 40 mg of labeled prussian blue insoluble, 99% of the administered prussian blue dose was excreted unchanged in feces. Absorption from multiple doses has not been studied.In a study using rats (n = 40, mean body weight range of 188 – 219 grams) injected with 137Cs, a dose response relationship was demonstrated for the amount of radiation elimination with prussian blue insoluble at doses of 1 to 50 mg/day (Table 1). There is little difference in radiation elimination rate between prussian blue insoluble at doses of 50 to 100 mg/day. In Table 1, the % of Injected Radiation Dose Remaining is defined as the percentage of the total injected dose of 137Cs remaining in the body at 96 hours post administration.
Table 1: Dose Response Relationship in Rats at 96 Hours
Prussian blue insoluble dose (mg/day)
% Injected 137Cs dose remaining (Range)
Untreated
58.1 (63.3 – 53.4)
1
9.42 (13.2 – 6.72)
10
1.17 (1.64 – 0.84)
50
0.57 (0.80 – 0.41)
100
0.52 (0.73 – 0.37)
In studies of rats, pigs, and dogs that were internally contaminated with cesium and thallium, the presence of the insoluble complexes in the gastrointestinal lumen changed the primary elimination route from the kidney to the feces and increased the rate of elimination of these two contaminants.
-
14 CLINICAL STUDIES
14.1 Cesium-137 Contamination
In literature reports, 72 people received Radiogardase after exposure to radioactive cesium ( 137Cs):
- 46 patients with 137Cs contamination
- 19 patients 137Cs contamination in other incidents
- 7 healthy human subjects who voluntarily ingested trace doses of 137Cs
In a 1987 incident in Goiânia, Brazil, 46 patients with heavy internal contamination with 137Cs were treated with Radiogardase (Table 2). Data on the whole body effective half-life of 137Cs, during and after Radiogardase treatment, was completed on 33 of these 46 patients (see Table 2). Radiogardase reduced the mean whole-body effective half-life of 137Cs by 69%, 46%, and 43% in adults, adolescents, and younger children, respectively.
Table 2 shows the decrease in whole body effective half-life of 137Cs in patients during Radiogardase treatment compared to the half-life of 137Cs after Radiogardase discontinuation (after treatment).
Table 2: Cesium-137 Effective Half-life During and After Treatment with Radiogardase
Group
Age
(years)
Radiogardase Dosage
137Cs Effective Half Life
During
Radiogardase TreatmentAfter Radiogardase Treatment
Adults (n=5)
> 18
10 grams/day
26 ± 6 days
80 ± 15 days
(all 21 adult patients)
Adults (n=10)
6 grams/day
25 ± 15 days
Adults (n=6)
3 grams/day
25 ± 9 days
Adolescents (n=5)
12 -14
< 10 grams/day
30 ± 12 days
62 ± 14 days
Children (n=7)
4 – 9
< 3 grams/day
24 ± 3 days
42 ± 4 days
Data from additional literature articles including 19 patients contaminated with 137Cs in other incidents and a study of 7 human subjects who voluntarily ingested trace doses of 137Cs showed a similar reduction in whole body effective half-life with Radiogardase treatment.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Radiogardase is supplied as gelatin capsules containing 0.5 grams of prussian blue insoluble for oral administration. The dark blue capsule is imprinted with the light blue inscription:
 PB. It is packaged in white plastic containers with a child-resistant tamper-evident closure. Each container contains 36 capsules.
PB. It is packaged in white plastic containers with a child-resistant tamper-evident closure. Each container contains 36 capsules.
- NDC: 58060-002-02
Storage
Store at 20 °C to 25 °C (68 °F to 77 °F), excursions permitted between 15 °C and 30°C (between 59 °F and 86 °F). Brief exposure to temperatures up to 40 °C (104 °F) may be tolerated, provided the mean kinetic temperature does not exceed 25 °C (77 °F); however, minimize such exposure. [see USP Controlled Room Temperature]
-
17 PATIENT COUNSELING INFORMATION
Decreased Gastrointestinal Motility
Inform patients that Radiogardase can decrease gastrointestinal motility. This can slow the transit time of cesium or thallium bound to Radiogardase and increase the radiation absorbed dose to the gastrointestinal mucosa. Alert patients to monitor for signs and symptoms of constipation and advise patients to seek medical management if symptoms develop.Precautions to Mitigate Radiation Exposure
Inform patients of safety measures to be taken to minimize radiation exposure to others or re-exposure to self. This includes instruction on appropriate use of the toilet, hand washing, and handling of items such as clothing that might get contaminated with body fluids.Discoloration of Stool, Oral Mucosa and Dentition
Inform patients taking Radiogardase that their stools might be blue-colored. Also inform patients that if the Radiogardase capsules are opened and the contents are mixed with food and eaten, the mouth and teeth may be colored blue.Manufactured by:
Haupt Pharma Wülfing GmbHDistribution by:
HEYL Chemisch-pharmazeutische
Fabrik GmbH & Co. KG,
Berlin - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RADIOGARDASE
prussian blue insoluble capsules capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:58060-002 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FERRIC FERROCYANIDE (UNII: TLE294X33A) (FERRIC FERROCYANIDE - UNII:TLE294X33A) FERRIC FERROCYANIDE 500 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) 38 mg GELATIN (UNII: 2G86QN327L) 83.12 mg WATER (UNII: 059QF0KO0R) 14.21 mg INDIGOTINDISULFONATE SODIUM (UNII: D3741U8K7L) 0.67 mg SODIUM LAURYL SULFATE (UNII: 368GB5141J) 0.15 mg Product Characteristics Color blue (Heyl;PB) Score no score Shape CAPSULE (Heyl;PB) Size 22mm Flavor Imprint Code Heyl;PB Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58060-002-02 1 in 1 CARTON 03/24/2010 1 36 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021626 03/24/2010 Labeler - Heyl Chem.-pharm. Fabrik GmbH & Co. KG (317151645) Registrant - Heyl Chem.-pharm. Fabrik GmbH & Co. KG (317151645) Establishment Name Address ID/FEI Business Operations Laborchemie Apolda GmbH 331821462 api manufacture(58060-002) Establishment Name Address ID/FEI Business Operations Ostthüringische Materialprüfgesellschaft für Textil- und Kunststoffe mbH 332338250 analysis(58060-002) Establishment Name Address ID/FEI Business Operations Haupt Pharma Wuelfing GmbH 333274975 manufacture(58060-002) , analysis(58060-002) , label(58060-002) , pack(58060-002) Establishment Name Address ID/FEI Business Operations SGS INSTITUT FRESENIUS GmbH 341259550 analysis(58060-002)



 PB
PB